Hidden impacts of ocean acidification to live and dead coral framework
- PMID: 26290073
- PMCID: PMC4632617
- DOI: 10.1098/rspb.2015.0990
Hidden impacts of ocean acidification to live and dead coral framework
Abstract
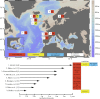
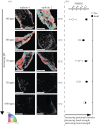
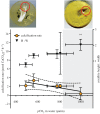
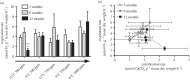
Cold-water corals, such as Lophelia pertusa, are key habitat-forming organisms found throughout the world's oceans to 3000 m deep. The complex three-dimensional framework made by these vulnerable marine ecosystems support high biodiversity and commercially important species. Given their importance, a key question is how both the living and the dead framework will fare under projected climate change. Here, we demonstrate that over 12 months L. pertusa can physiologically acclimate to increased CO2, showing sustained net calcification. However, their new skeletal structure changes and exhibits decreased crystallographic and molecular-scale bonding organization. Although physiological acclimatization was evident, we also demonstrate that there is a negative correlation between increasing CO2 levels and breaking strength of exposed framework (approx. 20-30% weaker after 12 months), meaning the exposed bases of reefs will be less effective 'load-bearers', and will become more susceptible to bioerosion and mechanical damage by 2100.
Keywords: Lophelia pertusa; biomineralization; calcification; climate change; cold-water corals; ocean acidification.
© 2015 The Authors.
Figures

References
-
- Roberts JM, Wheeler A, Freiwald A, Cairns SD. 2009. Cold-water corals: the biology and geology of deep-sea coral habitats. Cambridge, UK: Cambridge University Press.
-
- Henry L, Navas JM, Hennige S, Wicks LC, Roberts JM. 2013. Shark spawning grounds on cold-water coral reefs: a compelling case for protection of vulnerable marine ecosystems. Biol. Conserv. 161, 67–70. ( 10.1016/j.biocon.2013.03.002) - DOI
-
- United Nations. 2007. Oceans and the law of the sea: sustainable fisheries, including through the 1995 Agreement for the Implementation of the Provisions of the United Nations Convention on the Law of the Sea of 10 December 1982 relating to the Conservation and Management of Straddling Fish Stocks and Highly Migratory Fish Stocks, and related instruments, vol. 2167, A/RES/62/177. Washington, DC: United Nations.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
