Hepatocyte Growth Factor and MET Support Mouse Enteric Nervous System Development, the Peristaltic Response, and Intestinal Epithelial Proliferation in Response to Injury
- PMID: 26290232
- PMCID: PMC4540795
- DOI: 10.1523/JNEUROSCI.5267-14.2015
Hepatocyte Growth Factor and MET Support Mouse Enteric Nervous System Development, the Peristaltic Response, and Intestinal Epithelial Proliferation in Response to Injury
Abstract
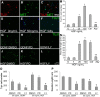
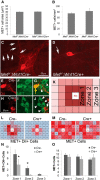
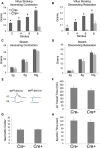
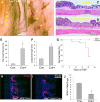
Factors providing trophic support to diverse enteric neuron subtypes remain poorly understood. We tested the hypothesis that hepatocyte growth factor (HGF) and the HGF receptor MET might support some types of enteric neurons. HGF and MET are expressed in fetal and adult enteric nervous system. In vitro, HGF increased enteric neuron differentiation and neurite length, but only if vanishingly small amounts (1 pg/ml) of glial cell line-derived neurotrophic factor were included in culture media. HGF effects were blocked by phosphatidylinositol-3 kinase inhibitor and by MET-blocking antibody. Both of these inhibitors and MEK inhibition reduced neurite length. In adult mice, MET was restricted to a subset of calcitonin gene-related peptide-immunoreactive (IR) myenteric plexus neurons thought to be intrinsic primary afferent neurons (IPANs). Conditional MET kinase domain inactivation (Met(fl/fl); Wnt1Cre+) caused a dramatic loss of myenteric plexus MET-IR neurites and 1-1'-dioctodecyl-3,3,3',3'-tetramethylindocarbocyamine perchlorate (DiI) labeling suggested reduced MET-IR neurite length. In vitro, Met(fl/fl); Wnt1Cre+ mouse bowel had markedly reduced peristalsis in response to mucosal deformation, but normal response to radial muscle stretch. However, whole-bowel transit, small-bowel transit, and colonic-bead expulsion were normal in Met(fl/fl); Wnt1Cre+ mice. Finally, Met(fl/fl); Wnt1Cre+ mice had more bowel injury and reduced epithelial cell proliferation compared with WT animals after dextran sodium sulfate treatment. These results suggest that HGF/MET signaling is important for development and function of a subset IPANs and that these cells regulate intestinal motility and epithelial cell proliferation in response to bowel injury.
Significance statement: The enteric nervous system has many neuronal subtypes that coordinate and control intestinal activity. Trophic factors that support these neuron types and enhance neurite growth after fetal development are not well understood. We show that a subset of adult calcitonin gene-related peptide (CGRP)-expressing myenteric neurons produce MET, the receptor for hepatocyte growth factor, and that loss of MET activity affects peristalsis in response to mucosal stroking, reduces MET-immunoreactive neurites, and increases susceptibility to dextran sodium sulfate-induced bowel injury. These observations may be relevant for understanding and treating intestinal motility disorders and also suggest that enhancing the activity of MET-expressing CGRP neurons might be a useful strategy to reduce bowel inflammation.
Keywords: MET; calcitonin gene-related peptide; dextran sodium sulfate (DSS); enteric nervous system; hepatocyte growth factor; intrinsic primary afferent neurons.
Copyright © 2015 the authors 0270-6474/15/3511544-16$15.00/0.
Figures




Similar articles
-
Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system.Gastroenterology. 2013 Dec;145(6):1323-33. doi: 10.1053/j.gastro.2013.08.047. Epub 2013 Aug 28. Gastroenterology. 2013. PMID: 23994200
-
MET Signaling Mediates Intestinal Crypt-Villus Development, Regeneration, and Adenoma Formation and Is Promoted by Stem Cell CD44 Isoforms.Gastroenterology. 2017 Oct;153(4):1040-1053.e4. doi: 10.1053/j.gastro.2017.07.008. Epub 2017 Jul 14. Gastroenterology. 2017. PMID: 28716720
-
Hepatocyte growth factor promotes colonic epithelial regeneration via Akt signaling.Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293(1):G230-9. doi: 10.1152/ajpgi.00068.2007. Epub 2007 Apr 5. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17412827
-
Neurotrophin-3 in the development of the enteric nervous system.Prog Brain Res. 2004;146:243-63. doi: 10.1016/S0079-6123(03)46016-0. Prog Brain Res. 2004. PMID: 14699968 Review.
-
Review article: serotonin receptors and transporters -- roles in normal and abnormal gastrointestinal motility.Aliment Pharmacol Ther. 2004 Nov;20 Suppl 7:3-14. doi: 10.1111/j.1365-2036.2004.02180.x. Aliment Pharmacol Ther. 2004. PMID: 15521849 Review.
Cited by
-
Anti-inflammatory effect of HGF responses to oral traumatic ulcers using an HGF-Tg mouse model.Exp Anim. 2022 May 20;71(2):204-213. doi: 10.1538/expanim.21-0141. Epub 2021 Nov 25. Exp Anim. 2022. PMID: 34819402 Free PMC article.
-
Molecular profiling of enteric nervous system cell lineages.Nat Protoc. 2022 Aug;17(8):1789-1817. doi: 10.1038/s41596-022-00697-4. Epub 2022 Jun 8. Nat Protoc. 2022. PMID: 35676375 Review.
-
Modulation of stem cell fate in intestinal homeostasis, injury and repair.World J Stem Cells. 2023 May 26;15(5):354-368. doi: 10.4252/wjsc.v15.i5.354. World J Stem Cells. 2023. PMID: 37342221 Free PMC article. Review.
-
Hirschsprung disease - integrating basic science and clinical medicine to improve outcomes.Nat Rev Gastroenterol Hepatol. 2018 Mar;15(3):152-167. doi: 10.1038/nrgastro.2017.149. Epub 2018 Jan 4. Nat Rev Gastroenterol Hepatol. 2018. PMID: 29300049 Review.
-
Enteric nervous system development: what could possibly go wrong?Nat Rev Neurosci. 2018 Sep;19(9):552-565. doi: 10.1038/s41583-018-0041-0. Nat Rev Neurosci. 2018. PMID: 30046054 Free PMC article. Review.
References
-
- Arthur LG, Schwartz MZ, Kuenzler KA, Birbe R. Hepatocyte growth factor treatment ameliorates diarrhea and bowel inflammation in a rat model of inflammatory bowel disease. J Pediatr Surg. 2004;39:139–143. discussion 139–143. - PubMed
-
- Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL, Gershon MD. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685–G695. doi: 10.1152/ajpgi.90685.2008. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
