Integration of New Information with Active Memory Accounts for Retrograde Amnesia: A Challenge to the Consolidation/Reconsolidation Hypothesis?
- PMID: 26290239
- PMCID: PMC6605236
- DOI: 10.1523/JNEUROSCI.1386-15.2015
Integration of New Information with Active Memory Accounts for Retrograde Amnesia: A Challenge to the Consolidation/Reconsolidation Hypothesis?
Abstract
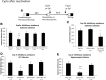
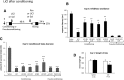
Active (new and reactivated) memories are considered to be labile and sensitive to treatments disrupting the time-dependent consolidation/reconsolidation processes required for their stabilization. Active memories also allow the integration of new information for updating memories. Here, we investigate the possibility that, when active, the internal state provided by amnesic treatments is represented and integrated within the initial memory and that amnesia results from the absence of this state at testing. We showed in rats that the amnesia resulting from systemic, intracerebroventricular and intrahippocampal injections of the protein synthesis inhibitor cycloheximide, administered after inhibitory avoidance training or reactivation, can be reversed by a reminder, including re-administration of the same drug. Similar results were obtained with lithium chloride (LiCl), which does not affect protein synthesis, when delivered systemically after training or reactivation. However, LiCl can induce memory given that a conditioned taste aversion was obtained for a novel taste, presented just before conditioning or reactivation. These results indicate that memories can be established and maintained without de novo protein synthesis and that experimental amnesia may not result from a disruption of memory consolidation/reconsolidation. The findings more likely support the integration hypothesis: posttraining/postreactivation treatments induce an internal state, which becomes encoded with the memory, and should be present at the time of testing to ensure a successful retrieval. This integration concept includes most of the previous explanations of memory recovery after retrograde amnesia and critically challenges the traditional memory consolidation/reconsolidation hypothesis, providing a more dynamic and flexible view of memory.
Significance statement: This study provides evidence challenging the traditional consolidation/reconsolidation hypotheses that have dominated the literature over the past 50 years. Based on amnesia studies, that hypothesis states that active (i.e., new and reactivated) memories are similarly labile and (re)established in a time-dependent manner within the brain through processes that require de novo protein synthesis. Our data show that new/reactivated memories can be formed without protein synthesis and that amnesia can be induced by drugs that do not affect protein synthesis. We propose that amnesia results from memory integration of the internal state produced by the drug that is subsequently necessary for retrieval of the memory. This interpretation gives a dynamic view of memory, rapidly stored and easily updated when active.
Keywords: amnesia; consolidation; malleability; memory reactivation; reconsolidation; state dependency.
Copyright © 2015 the authors 0270-6474/15/3511623-11$15.00/0.
Figures


Similar articles
-
Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval.Nature. 2000 Aug 17;406(6797):722-6. doi: 10.1038/35021052. Nature. 2000. PMID: 10963596
-
Memory integration: An alternative to the consolidation/reconsolidation hypothesis.Prog Neurobiol. 2018 Dec;171:15-31. doi: 10.1016/j.pneurobio.2018.10.002. Epub 2018 Oct 18. Prog Neurobiol. 2018. PMID: 30343034 Review.
-
Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms.PLoS Biol. 2005 Sep;3(9):e293. doi: 10.1371/journal.pbio.0030293. Epub 2005 Aug 23. PLoS Biol. 2005. PMID: 16104829 Free PMC article.
-
Memory Integration as a Challenge to the Consolidation/Reconsolidation Hypothesis: Similarities, Differences and Perspectives.Front Syst Neurosci. 2019 Jan 11;12:71. doi: 10.3389/fnsys.2018.00071. eCollection 2018. Front Syst Neurosci. 2019. PMID: 30687031 Free PMC article. Review.
-
Apparent reconsolidation interference without generalized amnesia.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Jun 8;108:110161. doi: 10.1016/j.pnpbp.2020.110161. Epub 2020 Nov 11. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 33186637 Free PMC article.
Cited by
-
Susceptibility of consolidated procedural memory to interference is independent of its active task-based retrieval.PLoS One. 2019 Jan 17;14(1):e0210876. doi: 10.1371/journal.pone.0210876. eCollection 2019. PLoS One. 2019. PMID: 30653576 Free PMC article.
-
Can Forgetting Be Due to Changes in Engram Cell Plasticity?Front Behav Neurosci. 2022 Jul 7;16:945985. doi: 10.3389/fnbeh.2022.945985. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35874646 Free PMC article. No abstract available.
-
The brain in motion: How ensemble fluidity drives memory-updating and flexibility.Elife. 2020 Dec 29;9:e63550. doi: 10.7554/eLife.63550. Elife. 2020. PMID: 33372892 Free PMC article. Review.
-
The computational nature of memory modification.Elife. 2017 Mar 15;6:e23763. doi: 10.7554/eLife.23763. Elife. 2017. PMID: 28294944 Free PMC article.
-
A comparison of behavioral and pharmacological interventions to attenuate reactivated fear memories.Learn Mem. 2017 Jul 17;24(8):369-374. doi: 10.1101/lm.045385.117. Print 2017 Aug. Learn Mem. 2017. PMID: 28716956 Free PMC article.
References
-
- Bradley PM, Galal KM. State-dependent recall can be induced by protein synthesis inhibition: behavioural and morphological observations. Brain Res. 1988;468:243–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
