Propofol: a review of its role in pediatric anesthesia and sedation
- PMID: 26290263
- PMCID: PMC4554966
- DOI: 10.1007/s40263-015-0259-6
Propofol: a review of its role in pediatric anesthesia and sedation
Erratum in
-
Correction to: Propofol: A Review of its Role in Pediatric Anesthesia and Sedation.CNS Drugs. 2018 Sep;32(9):873. doi: 10.1007/s40263-018-0561-1. CNS Drugs. 2018. PMID: 30101390
Abstract
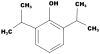
Propofol is an intravenous agent used commonly for the induction and maintenance of anesthesia, procedural, and critical care sedation in children. The mechanisms of action on the central nervous system involve interactions at various neurotransmitter receptors, especially the gamma-aminobutyric acid A receptor. Approved for use in the USA by the Food and Drug Administration in 1989, its use for induction of anesthesia in children less than 3 years of age still remains off-label. Despite its wide use in pediatric anesthesia, there is conflicting literature about its safety and serious adverse effects in particular subsets of children. Particularly as children are not "little adults", in this review, we emphasize the maturational aspects of propofol pharmacokinetics. Despite the myriad of propofol pharmacokinetic-pharmacodynamic studies and the ability to use allometrical scaling to smooth out differences due to size and age, there is no optimal model that can be used in target controlled infusion pumps for providing closed loop total intravenous anesthesia in children. As the commercial formulation of propofol is a nutrient-rich emulsion, the risk for bacterial contamination exists despite the Food and Drug Administration mandating addition of antimicrobial preservative, calling for manufacturers' directions to discard open vials after 6 h. While propofol has advantages over inhalation anesthesia such as less postoperative nausea and emergence delirium in children, pain on injection remains a problem even with newer formulations. Propofol is known to depress mitochondrial function by its action as an uncoupling agent in oxidative phosphorylation. This has implications for children with mitochondrial diseases and the occurrence of propofol-related infusion syndrome, a rare but seriously life-threatening complication of propofol. At the time of this review, there is no direct evidence in humans for propofol-induced neurotoxicity to the infant brain; however, current concerns of neuroapoptosis in developing brains induced by propofol persist and continue to be a focus of research.
Figures



References
-
- Trapani G, Altomare C, Liso G, Sanna E, Biggio G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Current medicinal chemistry. 2000;7(2):249–71. - PubMed
-
- Borgeat A, Popovic V, Meier D, Schwander D. Comparison of propofol and thiopental/halothane for short-duration ENT surgical procedures in children. Anesthesia and analgesia. 1990;71(5):511–5. - PubMed
-
- Reed MD, Yamashita TS, Marx CM, Myers CM, Blumer JL. A pharmacokinetically based propofol dosing strategy for sedation of the critically ill, mechanically ventilated pediatric patient. Critical care medicine. 1996;24(9):1473–81. - PubMed
-
- Reed MD, Blumer JL. Propofol bashing: the time to stop is now! Critical care medicine. 1996;24(1):175–6. - PubMed
-
- Angelini G, Ketzler JT, Coursin DB. Use of propofol and other nonbenzodiazepine sedatives in the intensive care unit. Critical care clinics. 2001;17(4):863–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

