Characterization of the putative phosphorylation sites of the AQP2 C terminus and their role in AQP2 trafficking in LLC-PK1 cells
- PMID: 26290367
- PMCID: PMC4609919
- DOI: 10.1152/ajprenal.00152.2015
Characterization of the putative phosphorylation sites of the AQP2 C terminus and their role in AQP2 trafficking in LLC-PK1 cells
Abstract
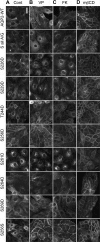
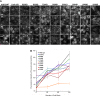
Vasopressin (VP) stimulates a signaling cascade that results in phosphorylation and apical membrane accumulation of aquaporin-2 (AQP2), leading to water reabsorption by kidney collecting ducts. However, the roles of most C-terminal phosphorylation events in stimulated and constitutive AQP2 recycling are incompletely understood. Here, we generated LLC-PK1 cells containing point mutations of all potential phosphorylation sites in the AQP2 C terminus: S226, S229, T244, S256, S261, S264, and S269, to determine their impact on AQP2 trafficking. We produced an All Null AQP2 construct in which these serine (S) or threonine (T) residues were mutated to alanine (A) or glycine (G), and we then reintroduced the phosphorylation mimic aspartic acid (D) individually to each site in the All Null mutant. As expected, the All Null mutant does not accumulate at the plasma membrane in response to VP but still undergoes constitutive recycling, as shown by its membrane accumulation when endocytosis is blocked by methyl-β-cyclodextrin (MβCD), and accumulation in a perinuclear patch at low temperature (20°C). Single phosphorylation mimics S226D, S229D, T244D, S261D, S264D, and S269D were insufficient to cause membrane accumulation of AQP2 alone or after VP treatment. However, AQP2 S256 reintroduced into the All Null mutant maintains its trafficking response to VP. We conclude that 1) constitutive recycling of AQP2 does not require phosphorylation at any C-terminal sites; 2) forced "phosphorylation" of sites in the AQP2 C terminus is insufficient to stimulate membrane accumulation in the absence of S256 phosphorylation; and 3) phosphorylation of S256 alone is necessary and sufficient to cause membrane accumulation of AQP2.
Keywords: LLC-PK1; aquaporin-2; endocytosis; phosphorylation; vasopressin.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

