Treatment of systemic lupus erythematosus patients with the BAFF antagonist "peptibody" blisibimod (AMG 623/A-623): results from randomized, double-blind phase 1a and phase 1b trials
- PMID: 26290435
- PMCID: PMC4545922
- DOI: 10.1186/s13075-015-0741-z
Treatment of systemic lupus erythematosus patients with the BAFF antagonist "peptibody" blisibimod (AMG 623/A-623): results from randomized, double-blind phase 1a and phase 1b trials
Abstract
Introduction: Blisibimod is a potent B cell-activating factor (BAFF) antagonist that binds to both cell membrane-expressed and soluble BAFF. The goal of these first-in-human studies was to characterize the safety, tolerability, and pharmacokinetic and pharmacodynamic profiles of blisibimod in subjects with systemic lupus erythematosus (SLE).
Methods: SLE subjects with mild disease that was stable/inactive at baseline received either a single dose of blisibimod (0.1, 0.3, 1, or 3 mg/kg subcutaneous [SC] or 1, 3, or 6 mg/kg intravenous [IV]) or placebo (phase 1a; N = 54), or four weekly doses of blisibimod (0.3, 1, or 3 mg/kg SC or 6 mg/kg IV) or placebo (phase 1b; N = 63). Safety and tolerability measures were collected, and B cell subset measurements and pharmacokinetic analyses were performed.
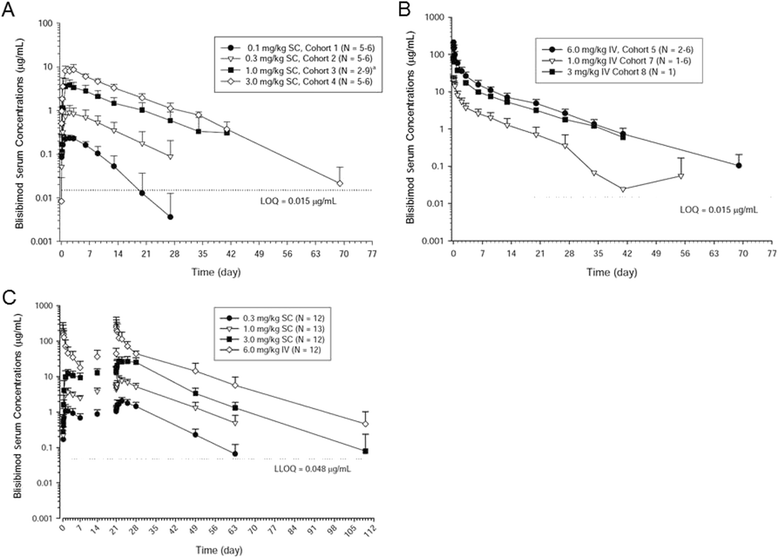
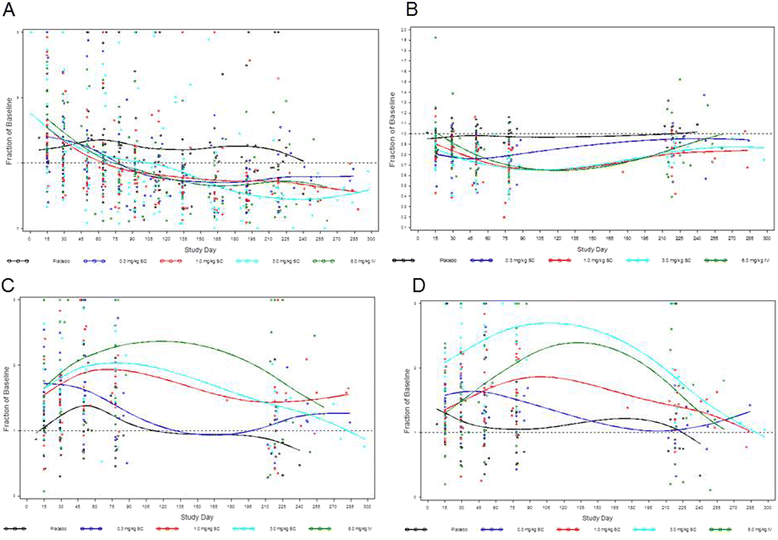
Results: All subjects (93 % female; mean age 43.7 years) carried the diagnosis of SLE for ≥ 1 year. Single- and multiple-dose treatment with blisibimod produced a decrease in the number of naïve B cells (24-76 %) and a transient relative increase in the memory B cell compartment, with the greatest effect on IgD(-)CD27+; there were no notable changes in T cells or natural killer cells. With time, memory B cells reverted to baseline, leading to a calculated 30 % reduction in total B cells by approximately 160 days after the first dose. In both the single- and multiple-dosing SC cohorts, the pharmacokinetic profile indicated slow absorption, dose-proportional exposure from 0.3 through 3.0 mg/kg SC and 1 through 6 mg/kg IV, linear pharmacokinetics across the dose range of 1.0-6.0 mg/kg, and accumulation ratios ranging from 2.21 to 2.76. The relative increase in memory B cells was not associated with safety signals, and the incidence of adverse events, anti-blisibimod antibodies, and clinical laboratory abnormalities were comparable between blisibimod- and placebo-treated subjects.
Conclusions: Blisibimod changed the constituency of the B cell pool and single and multiple doses of blisibimod exhibited approximate dose-proportional pharmacokinetics across the dose range 1.0-6.0 mg/kg. The safety and tolerability profile of blisibimod in SLE was comparable with that of placebo. These findings support further studies of blisibimod in SLE and other B cell-mediated diseases.
Trial registration: Clinicaltrials.gov NCT02443506 . Registered 11 May 2015. NCT02411136 Registered 7 April 2015.
Figures
Similar articles
-
Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial.Ann Rheum Dis. 2018 Jun;77(6):883-889. doi: 10.1136/annrheumdis-2018-213032. Epub 2018 Mar 21. Ann Rheum Dis. 2018. PMID: 29563108 Clinical Trial.
-
A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study.Ann Rheum Dis. 2015 Sep;74(9):1667-75. doi: 10.1136/annrheumdis-2013-205144. Epub 2014 Apr 19. Ann Rheum Dis. 2015. PMID: 24748629 Clinical Trial.
-
Spotlight on blisibimod and its potential in the treatment of systemic lupus erythematosus: evidence to date.Drug Des Devel Ther. 2017 Mar 13;11:747-757. doi: 10.2147/DDDT.S114552. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28331294 Free PMC article. Review.
-
Blisibimod for treatment of systemic lupus erythematosus: with trials you become wiser.Expert Opin Biol Ther. 2016;16(5):723-33. doi: 10.1517/14712598.2016.1169270. Expert Opin Biol Ther. 2016. PMID: 27051973 Review.
-
Assessments of fatigue and disease activity in patients with systemic lupus erythematosus enrolled in the Phase 2 clinical trial with blisibimod.Lupus. 2017 Jan;26(1):27-37. doi: 10.1177/0961203316654767. Epub 2016 Jun 26. Lupus. 2017. PMID: 27353505 Clinical Trial.
Cited by
-
B Cell Aberrance in Lupus: the Ringleader and the Solution.Clin Rev Allergy Immunol. 2022 Apr;62(2):301-323. doi: 10.1007/s12016-020-08820-7. Epub 2021 Feb 3. Clin Rev Allergy Immunol. 2022. PMID: 33534064 Review.
-
Clinical Pharmacokinetics and Pharmacodynamics of Biologic Therapeutics for Treatment of Systemic Lupus Erythematosus.Clin Pharmacokinet. 2017 Feb;56(2):107-125. doi: 10.1007/s40262-016-0426-z. Clin Pharmacokinet. 2017. PMID: 27384528 Free PMC article. Review.
-
Reductions in circulating B cell subsets and immunoglobulin G levels with long-term belimumab treatment in patients with SLE.Lupus Sci Med. 2022 Feb;9(1):e000499. doi: 10.1136/lupus-2021-000499. Lupus Sci Med. 2022. PMID: 35131846 Free PMC article. Clinical Trial.
-
Overview of pathophysiology and treatment of human lupus nephritis.Curr Opin Rheumatol. 2016 Sep;28(5):460-7. doi: 10.1097/BOR.0000000000000319. Curr Opin Rheumatol. 2016. PMID: 27341623 Free PMC article. Review.
-
Recent advances in the management of systemic lupus erythematosus.F1000Res. 2018 Jun 29;7:F1000 Faculty Rev-970. doi: 10.12688/f1000research.13941.1. eCollection 2018. F1000Res. 2018. PMID: 30026918 Free PMC article. Review.
References
-
- Thompson J, Schneider P, Kalled S, Wang L, Lefevre E, Cachero T, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192:129–135. doi: 10.1084/jem.192.1.129. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

