Rapid Histone-Catalyzed DNA Lesion Excision and Accompanying Protein Modification in Nucleosomes and Nucleosome Core Particles
- PMID: 26290445
- PMCID: PMC4612368
- DOI: 10.1021/jacs.5b05478
Rapid Histone-Catalyzed DNA Lesion Excision and Accompanying Protein Modification in Nucleosomes and Nucleosome Core Particles
Abstract
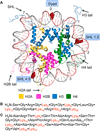
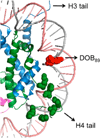
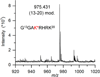
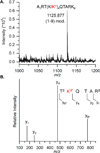
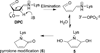
C5'-Hydrogen atoms are frequently abstracted during DNA oxidation. The oxidized abasic lesion 5'-(2-phosphoryl-1,4-dioxobutane) (DOB) is an electrophilic product of the C5'-radical. DOB is a potent irreversible inhibitor of DNA polymerase β, and forms interstrand cross-links in free DNA. We examined the reactivity of DOB within nucleosomes and nucleosome core particles (NCPs), the monomeric component of chromatin. Depending upon the position at which DOB is generated within a NCP, it is excised from nucleosomal DNA at a rate 275-1500-fold faster than that in free DNA. The half-life of DOB (7.0-16.8 min) in NCPs is shorter than any other abasic lesion. DOB's lifetime in NCPs is also significantly shorter than the estimated lifetime of an abasic site within a cell, suggesting that the observed chemistry would occur intracellularly. Histones also catalyze DOB excision when the lesion is present in the DNA linker region of a nucleosome. Schiff-base formation between DOB and histone proteins is detected in nucleosomes and NCPs, resulting in pyrrolone formation at the lysine residues. The lysines modified by DOB are often post-translationally modified. Consequently, the histone modifications described herein could affect the regulation of gene expression and may provide a chemical basis for the cytotoxicity of the DNA damaging agents that produce this lesion.
Figures











Similar articles
-
Irreversible inhibition of DNA polymerase beta by an oxidized abasic lesion.J Am Chem Soc. 2010 Apr 14;132(14):5004-5. doi: 10.1021/ja101372c. J Am Chem Soc. 2010. PMID: 20334373 Free PMC article.
-
Reactivity of the Major Product of C5'-Oxidative DNA Damage in Nucleosome Core Particles.Chembiochem. 2019 Mar 1;20(5):672-676. doi: 10.1002/cbic.201800663. Epub 2019 Jan 10. Chembiochem. 2019. PMID: 30444560 Free PMC article.
-
DNA polymerase λ inactivation by oxidized abasic sites.Biochemistry. 2013 Feb 5;52(5):975-83. doi: 10.1021/bi301592x. Epub 2013 Jan 18. Biochemistry. 2013. PMID: 23330920 Free PMC article.
-
Structure and dynamic properties of nucleosome core particles.FEBS Lett. 2005 Feb 7;579(4):895-8. doi: 10.1016/j.febslet.2004.11.030. FEBS Lett. 2005. PMID: 15680970 Review.
-
A modified epigenetics toolbox to study histone modifications on the nucleosome core.Chembiochem. 2011 Jan 24;12(2):308-13. doi: 10.1002/cbic.201000617. Epub 2010 Dec 29. Chembiochem. 2011. PMID: 21243718 Review.
Cited by
-
Effect of Histone Lysine Methylation on DNA Lesion Reactivity in Nucleosome Core Particles.Chem Res Toxicol. 2019 May 20;32(5):910-916. doi: 10.1021/acs.chemrestox.9b00049. Epub 2019 Mar 27. Chem Res Toxicol. 2019. PMID: 30916939 Free PMC article.
-
Nucleosome Histone Tail Conformation and Dynamics: Impacts of Lysine Acetylation and a Nearby Minor Groove Benzo[a]pyrene-Derived Lesion.Biochemistry. 2017 Apr 11;56(14):1963-1973. doi: 10.1021/acs.biochem.6b01208. Epub 2017 Mar 22. Biochemistry. 2017. PMID: 28304160 Free PMC article.
-
5-Formylcytosine Yields DNA-Protein Cross-Links in Nucleosome Core Particles.J Am Chem Soc. 2017 Aug 9;139(31):10617-10620. doi: 10.1021/jacs.7b05495. Epub 2017 Jul 25. J Am Chem Soc. 2017. PMID: 28742335 Free PMC article.
-
Quantification of Serum High Mobility Group Box 1 by Liquid Chromatography/High-Resolution Mass Spectrometry: Implications for Its Role in Immunity, Inflammation, and Cancer.Anal Chem. 2018 Jun 19;90(12):7552-7560. doi: 10.1021/acs.analchem.8b01175. Epub 2018 Jun 5. Anal Chem. 2018. PMID: 29791130 Free PMC article.
-
Stable Interstrand Cross-Links Generated from the Repair of 1,N6-Ethenoadenine in DNA by α-Ketoglutarate/Fe(II)-Dependent Dioxygenase ALKBH2.J Am Chem Soc. 2024 Apr 17;146(15):10381-10392. doi: 10.1021/jacs.3c12890. Epub 2024 Apr 4. J Am Chem Soc. 2024. PMID: 38573229 Free PMC article.
References
-
- Dizdaroglu M. Mutat. Res., Rev. Mutat. Res. 2015;763:212–245. - PubMed
-
- Rabow LE, Stubbe J, Kozarich JW. J. Am. Chem. Soc. 1990;112:3196–3203.
-
- Rabow LE, McGall GH, Stubbe J, Kozarich JW. J. Am. Chem. Soc. 1990;112:3203–3208.
-
- Aso M, Kondo M, Suemune H, Hecht SM. J. Am. Chem. Soc. 1999;121:9023–9033.
-
- Sugiyama H, Xu C, Murugesan N, Hecht SM. J. Am. Chem. Soc. 1985;107:4104–4105.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

