The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging
- PMID: 26290460
- PMCID: PMC4693467
- DOI: 10.1111/acel.12387
The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging
Abstract
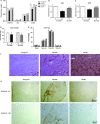
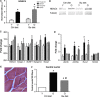
Sarcopenia, the loss of skeletal muscle mass and function during aging, is a major contributor to disability and frailty in the elderly. Previous studies found a protective effect of reduced histone deacetylase activity in models of neurogenic muscle atrophy. Because loss of muscle mass during aging is associated with loss of motor neuron innervation, we investigated the potential for the histone deacetylase (HDAC) inhibitor butyrate to modulate age-related muscle loss. Consistent with previous studies, we found significant loss of hindlimb muscle mass in 26-month-old C57Bl/6 female mice fed a control diet. Butyrate treatment starting at 16 months of age wholly or partially protected against muscle atrophy in hindlimb muscles. Butyrate increased muscle fiber cross-sectional area and prevented intramuscular fat accumulation in the old mice. In addition to the protective effect on muscle mass, butyrate reduced fat mass and improved glucose metabolism in 26-month-old mice as determined by a glucose tolerance test. Furthermore, butyrate increased markers of mitochondrial biogenesis in skeletal muscle and whole-body oxygen consumption without affecting activity. The increase in mass in butyrate-treated mice was not due to reduced ubiquitin-mediated proteasomal degradation. However, butyrate reduced markers of oxidative stress and apoptosis and altered antioxidant enzyme activity. Our data is the first to show a beneficial effect of butyrate on muscle mass during aging and suggests HDACs contribute to age-related muscle atrophy and may be effective targets for intervention in sarcopenia and age-related metabolic disease.
Keywords: aging; butyrate; histone deacetylase; metabolism; sarcopenia; skeletal muscle.
© 2015 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures




Similar articles
-
Effect of antioxidant supplementation on skeletal muscle and metabolic profile in aging mice.Food Funct. 2021 Jan 21;12(2):825-833. doi: 10.1039/d0fo02051f. Epub 2021 Jan 5. Food Funct. 2021. PMID: 33399617
-
TRIM16 facilitates SIRT-1-dependent regulation of antioxidant response to alleviate age-related sarcopenia.J Cachexia Sarcopenia Muscle. 2024 Oct;15(5):2056-2070. doi: 10.1002/jcsm.13553. Epub 2024 Aug 27. J Cachexia Sarcopenia Muscle. 2024. PMID: 39192479 Free PMC article.
-
Parkin overexpression protects from ageing-related loss of muscle mass and strength.J Physiol. 2019 Apr;597(7):1975-1991. doi: 10.1113/JP277157. Epub 2019 Jan 30. J Physiol. 2019. PMID: 30614532 Free PMC article.
-
Emerging roles for histone deacetylases in age-related muscle atrophy.Nutr Healthy Aging. 2016 Oct 27;4(1):17-30. doi: 10.3233/NHA-160005. Nutr Healthy Aging. 2016. PMID: 28035339 Free PMC article. Review.
-
Redox Systems, Antioxidants and Sarcopenia.Curr Protein Pept Sci. 2018;19(7):643-648. doi: 10.2174/1389203718666170317120040. Curr Protein Pept Sci. 2018. PMID: 28317484 Review.
Cited by
-
Changes in the gut microbiota of patients with sarcopenia based on 16S rRNA gene sequencing: a systematic review and meta-analysis.Front Nutr. 2024 Jun 28;11:1429242. doi: 10.3389/fnut.2024.1429242. eCollection 2024. Front Nutr. 2024. PMID: 39006102 Free PMC article.
-
Evidence for the Contribution of Gut Microbiota to Age-Related Anabolic Resistance.Nutrients. 2021 Feb 23;13(2):706. doi: 10.3390/nu13020706. Nutrients. 2021. PMID: 33672207 Free PMC article. Review.
-
Role of the Gut Microbiome in Skeletal Muscle Physiology and Pathophysiology.Curr Osteoporos Rep. 2022 Dec;20(6):422-432. doi: 10.1007/s11914-022-00752-9. Epub 2022 Sep 19. Curr Osteoporos Rep. 2022. PMID: 36121571 Review.
-
Gut Microbiome and Space Travelers' Health: State of the Art and Possible Pro/Prebiotic Strategies for Long-Term Space Missions.Front Physiol. 2020 Sep 8;11:553929. doi: 10.3389/fphys.2020.553929. eCollection 2020. Front Physiol. 2020. PMID: 33013480 Free PMC article. Review.
-
HDAC8 regulates protein kinase D phosphorylation in skeletal myoblasts in response to stress signaling.Biochem Biophys Res Commun. 2023 Apr 2;650:81-86. doi: 10.1016/j.bbrc.2023.02.010. Epub 2023 Feb 6. Biochem Biophys Res Commun. 2023. PMID: 36773343 Free PMC article.
References
-
- Balagopal P, Rooyackers O, Adey D, Ades P, Nair S (1997) Effects of aging on in vivo synthesis of skeletal muscle myosin heavy‐chain and sarcoplasmic protein in humans. Am. J. Physiol. 273, E790–E800. - PubMed
-
- Briguet A, Courdier‐Fruh I, Foster M, Meier T, Magyar J (2004) Histological parameters for the quantitative assessment of muscular dystrophy in the mdx‐mouse. Neuromuscul. Disord. 14, 675–682. - PubMed
-
- Consalvi S, Mozzetta C, Bettica P, Germani M, Fiorentini F, Del Bene F, Rocchetti M, Leoni F, Monzani V, Mascagni P, Puri PL, Saccone V (2013) Preclinical studies in the mdx mouse model of duchenne muscular dystrophy with the histone deacetylase inhibitor givinostat. Mol. Med. 19, 79–87. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

