Nonmuscle myosin heavy chain IIA mediates Epstein-Barr virus infection of nasopharyngeal epithelial cells
- PMID: 26290577
- PMCID: PMC4568263
- DOI: 10.1073/pnas.1513359112
Nonmuscle myosin heavy chain IIA mediates Epstein-Barr virus infection of nasopharyngeal epithelial cells
Abstract
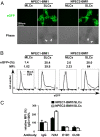
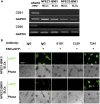
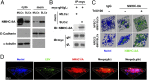
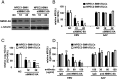
EBV causes B lymphomas and undifferentiated nasopharyngeal carcinoma (NPC). Although the mechanisms by which EBV infects B lymphocytes have been extensively studied, investigation of the mechanisms by which EBV infects nasopharyngeal epithelial cells (NPECs) has only recently been enabled by the successful growth of B lymphoma Mo-MLV insertion region 1 homolog (BMI1)-immortalized NPECs in vitro and the discovery that neuropilin 1 expression positively affects EBV glycoprotein B (gB)-mediated infection and tyrosine kinase activations in enhancing EBV infection of BMI1-immortalized NPECs. We have now found that even though EBV infected NPECs grown as a monolayer at extremely low efficiency (<3%), close to 30% of NPECs grown as sphere-like cells (SLCs) were infected by EBV. We also identified nonmuscle myosin heavy chain IIA (NMHC-IIA) as another NPEC protein important for efficient EBV infection. EBV gH/gL specifically interacted with NMHC-IIA both in vitro and in vivo. NMHC-IIA densely aggregated on the surface of NPEC SLCs and colocalized with EBV. EBV infection of NPEC SLCs was significantly reduced by NMHC-IIA siRNA knock-down. NMHC-IIA antisera also efficiently blocked EBV infection. These data indicate that NMHC-IIA is an important factor for EBV NPEC infection.
Keywords: BMI1; Epstein–Barr virus; NMHC-IIA; gH/gL; nasopharyngeal carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

