E-cadherin junction formation involves an active kinetic nucleation process
- PMID: 26290581
- PMCID: PMC4568248
- DOI: 10.1073/pnas.1513775112
E-cadherin junction formation involves an active kinetic nucleation process
Erratum in
-
Correction for Biswas et al., E-cadherin junction formation involves an active kinetic nucleation process.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):E7640. doi: 10.1073/pnas.1617360113. Epub 2016 Nov 14. Proc Natl Acad Sci U S A. 2016. PMID: 27849591 Free PMC article. No abstract available.
-
Correction to Supporting Information for Biswas et al., E-cadherin junction formation involves an active kinetic nucleation process.Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):E7870. doi: 10.1073/pnas.1617361113. Epub 2016 Nov 14. Proc Natl Acad Sci U S A. 2016. PMID: 27849605 Free PMC article. No abstract available.
Abstract
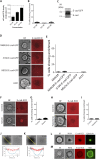
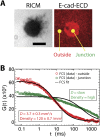
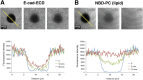
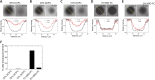
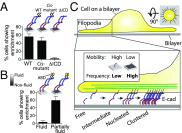
Epithelial (E)-cadherin-mediated cell-cell junctions play important roles in the development and maintenance of tissue structure in multicellular organisms. E-cadherin adhesion is thus a key element of the cellular microenvironment that provides both mechanical and biochemical signaling inputs. Here, we report in vitro reconstitution of junction-like structures between native E-cadherin in living cells and the extracellular domain of E-cadherin (E-cad-ECD) in a supported membrane. Junction formation in this hybrid live cell-supported membrane configuration requires both active processes within the living cell and a supported membrane with low E-cad-ECD mobility. The hybrid junctions recruit α-catenin and exhibit remodeled cortical actin. Observations suggest that the initial stages of junction formation in this hybrid system depend on the trans but not the cis interactions between E-cadherin molecules, and proceed via a nucleation process in which protrusion and retraction of filopodia play a key role.
Keywords: adhesion; bilayer; cadherin; diffusion; nucleation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

