Awake fMRI reveals a specialized region in dog temporal cortex for face processing
- PMID: 26290784
- PMCID: PMC4540004
- DOI: 10.7717/peerj.1115
Awake fMRI reveals a specialized region in dog temporal cortex for face processing
Abstract
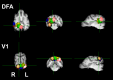
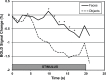
Recent behavioral evidence suggests that dogs, like humans and monkeys, are capable of visual face recognition. But do dogs also exhibit specialized cortical face regions similar to humans and monkeys? Using functional magnetic resonance imaging (fMRI) in six dogs trained to remain motionless during scanning without restraint or sedation, we found a region in the canine temporal lobe that responded significantly more to movies of human faces than to movies of everyday objects. Next, using a new stimulus set to investigate face selectivity in this predefined candidate dog face area, we found that this region responded similarly to images of human faces and dog faces, yet significantly more to both human and dog faces than to images of objects. Such face selectivity was not found in dog primary visual cortex. Taken together, these findings: (1) provide the first evidence for a face-selective region in the temporal cortex of dogs, which cannot be explained by simple low-level visual feature extraction; (2) reveal that neural machinery dedicated to face processing is not unique to primates; and (3) may help explain dogs' exquisite sensitivity to human social cues.
Keywords: Dog; Face area; fMRI.
Conflict of interest statement
Mark Spivak is president of Comprehensive Pet Therapy. Gregory Berns and Mark Spivak own equity in Dog Star Technologies and developed technology used in the research described in this paper. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Figures


References
-
- Beitz AJ, Fletcher TF. The brain. In: Evans HE, editor. Miller’s anatomy of the dog. Third Edition. Philadelphia: W.B. Saunders Company; 1993.
-
- Bruce V, Young A. In the eye of the beholder: the science of face perception. New York: Oxford University Press; 1998.
LinkOut - more resources
Full Text Sources
Other Literature Sources

