Peripheral Administration of Tumor Necrosis Factor-Alpha Induces Neuroinflammation and Sickness but Not Depressive-Like Behavior in Mice
- PMID: 26290874
- PMCID: PMC4531164
- DOI: 10.1155/2015/716920
Peripheral Administration of Tumor Necrosis Factor-Alpha Induces Neuroinflammation and Sickness but Not Depressive-Like Behavior in Mice
Abstract
Clinical observations indicate that activation of the TNF-α system may contribute to the development of inflammation-associated depression. Here, we tested the hypothesis that systemic upregulation of TNF-α induces neuroinflammation and behavioral changes relevant to depression. We report that a single intraperitoneal injection of TNF-α in mice increased serum and brain levels of the proinflammatory mediators TNF-α, IL-6, and MCP-1, in a dose- and time-dependent manner, but not IL-1β. Protein levels of the anti-inflammatory cytokine IL-10 increased in serum but not in the brain. The transient release of immune molecules was followed by glial cell activation as indicated by increased astrocyte activation in bioluminescent Gfap-luc mice and elevated immunoreactivity against the microglial marker Iba1 in the dentate gyrus of TNF-α-challenged mice. Additionally, TNF-α-injected mice were evaluated in a panel of behavioral tests commonly used to study sickness and depressive-like behavior in rodents. Our behavioral data imply that systemic administration of TNF-α induces a strong sickness response characterized by reduced locomotor activity, decreased fluid intake, and body weight loss. Depressive-like behavior could not be separated from sickness at any of the time points studied. Together, these results demonstrate that peripheral TNF-α affects the central nervous system at a neuroimmune and behavioral level.
Figures
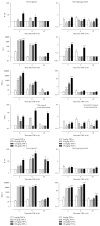


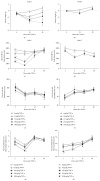

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

