Altered Neuronal and Circuit Excitability in Fragile X Syndrome
- PMID: 26291156
- PMCID: PMC4545495
- DOI: 10.1016/j.neuron.2015.06.017
Altered Neuronal and Circuit Excitability in Fragile X Syndrome
Abstract
Fragile X syndrome (FXS) results from a genetic mutation in a single gene yet produces a phenotypically complex disorder with a range of neurological and psychiatric problems. Efforts to decipher how perturbations in signaling pathways lead to the myriad alterations in synaptic and cellular functions have provided insights into the molecular underpinnings of this disorder. From this large body of data, the theme of circuit hyperexcitability has emerged as a potential explanation for many of the neurological and psychiatric symptoms in FXS. The mechanisms for hyperexcitability range from alterations in the expression or activity of ion channels to changes in neurotransmitters and receptors. Contributions of these processes are often brain region and cell type specific, resulting in complex effects on circuit function that manifest as altered excitability. Here, we review the current state of knowledge of the molecular, synaptic, and circuit-level mechanisms underlying hyperexcitability and their contributions to the FXS phenotypes.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

- (A)
Audiogenic seizures (AGS) are easier to elicit in Fmr1 KO mice of the FVB background. Percentage of animals showing AGS (light bars) and percentage mortality from status epilepticus (dark bars) after exposure to a high intensity siren delivering an average sound pressure level of 125 dB at 11cm for up to 15 min. [Adapted from Yan et al., 2005]
- (B)
Auditory startle is enhanced in Fmr1 KO mice. Fmr1 KO mice in c57 background (and FVB, not shown) show greater startle amplitude responses (measured as whole-body flinches) to low intensity acoustic stimuli (<90 dB) and lower responses to high intensity stimuli (>110 dB) compared to WT mice at 12–15 weeks of age. (from Nielsen et al., 2002)
- (C)
Whole-body startle response to 20 ms auditory stimuli (values over 65 dB background white noise) is exaggerated in 9–16 week-old Fmr1−/y mice compared to WT mice. [from Zhang et al., 2014]
- (D)
Disrupted circadian rhythms in Fmr1 KO mice. Left, Representative locomotor activity records of WT and KO mice. Activity records are double-plotted. Times of activity (wheel running) are indicated by black vertical marks, during exposure to a 12:12 light/dark cycle and after release into constant darkness (LD/DD). Right, Enlarged record showing clear disruption of circadian locomotor activity in the mutant animal, including frequent awakenings during the day. [from Zhang et al., 2008]

- (A)
Chart representing various aspects of neuronal excitability affected by FMRP loss and corresponding changes in the absence of FMRP in expression of proteins regulating these processes. Interactions that have been functionally validated and demonstrated to have an effect on excitability in Fmr1 KO mice are shown in BOLD.
- (B-C)
FMRP regulates dendritic excitability.
- (B)
Dendritic sag associated with the Ih current in Fmr1 KO mice is either higher (Left, hippocampus) or lower (Right, neocortex) than in WT mice. [from Brager et al., 2012 and Zhang et al., 2014, respectively]
- (C)
Back-propagating APs are larger in dendrites of hippocampal pyramidal neurons of Fmr1 KO mice due to a reduction in dendritic A-type K+ current (insert) [from Routh et al., 2013].
- (D–G)
FMRP regulates neuronal excitability via direct modulation of ion channel properties independently of its role in translational regulation.
- (D)
FMRP binds to and directly regulates gating of a K+ channel Slack [from Brown et al., 2010].
- (E)
FMRP directly regulates activity of BK channels via interactions with the channel auxiliary β4 subunit. As a result AP duration is longer in hippocampal and cortical excitatory neurons of Fmr1 KO mice [from Deng et al. 2013].
- (F)
FMRP missense mutation R138Q found in a patient with a partial FXS (intellectual disability and seizures) strongly reduces FMRP-BK β4 subunit interactions and renders FMRP unable to regulate AP duration (from Myrick et al., 2015).
- (G)
FMRP directly binds to and regulates surface expression of presynaptic N-type Ca2+ channels in DRG neurons [from Ferron et al., 2014].

- (A)
Changes in synaptic strength during natural stimulus trains plotted as a function of stimulus number for WT and Fmr1 KO mice. Inset shows EPSCs 75–83 during the natural stimulus trains, scaled to their own controls for comparison. (from Deng et al., 2011).
- (B)
Dendritic input resistance is increased in cortical L5 pyramidal neurons of Fmr1 KO mice [from Zhang et al., 2014].
- (C)
Delayed GABA polarity switch. Left, ECl- remains depolarized in Fmr1 KO mice during cortical development. Average ECl- calculated from individual recordings plotted against the age of the mouse. The resting membrane potential (RMP) measured at P10 is denoted by the dashed line and shaded area represents points at which GABA would have a mature hyperpolarizing response. *p < 0.05 [from He et al., 2014]. Right, Age-dependence of the driving force of GABA-A receptor (DFGABA) in neurons from control and Fmr1 KO mice [from Tyzio et al., 2014]
- (D)
Spontaneous activity is increased in Fmr1 KO mice at P15. Left, representative traces of whole-cell voltage clamp recordings of sEPSCs at –70 mV from individual hippocampal CA3 pyramidal neurons in acute brain slices. Right, Average values of sEPSC frequencies are higher in Fmr1 KO mice, but are normalized by treatment with bumetanide at birth. [from Tyzio et al., 2014]
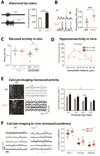
- (A)
Left, Representative extracellular multiunit recordings from L4 in slices from barrel cortex of WT and Fmr1−/y mice. Right, Group averages showing prolonged UP state duration in slices from Fmr1−/y mice (n = 22). [from Ronesi et al., 2012]
- (B)
Left, Sample traces from whole-cell patch-clamp in vivo recordings of L2/3 neurons during UP/DOWN states in unanesthetized WT and Fmr1−/− mice. Right, The mean firing probability during any given Up state (active or silent) was higher in Fmr1−/− mice (**p < 0.01, t-test). In contrast, the frequency and duration of Up states were the same in WT and mutant mice (not shown). [from Goncalves et al., 2013]
- (C)
Firing rates for L2/3 neurons are higher in Fmr1−/− mice compared to WT mice during in vivo whole-cell recordings showing UP/DOWN states (typical of sleep or quiet wakefulness), but not during fast oscillatory activity (FOA; typical of awake brain state). *p < 0.05. [from Goncalves et al., 2013]
- (D)
Hyperconnectivity of L5 pyramidal neurons in prefrontal cortex of 2–3 week old Fmr1 KO mice. Direct connections between neurons were tested at a range of distances using hexa-patch electrode recordings in brain slices. Connection probability distributions were significantly higher for clusters of Fmr1 KO neurons than for those of WT neurons (p < 0.01). The same analysis in 3–5 week-old mice did not reveal any significant differences. (from Testa-Silva et al., 2011)
- (E)
Hyperactive postnatal brain networks in Fmr1 KO mice. Left, representative two-photon images of acute coronal sections of the cortex at P4 from WT and Fmr1 KO mice that were loaded with the fluorescent calcium indicator Fura2-AM and corresponding sample traces for 4 neurons. Arrows indicate epochs of synchronous firing in the recording. Right, frequency of activity during development in WT and Fmr1 KO mice (**p = 0.024 across ages, and *p = 0.002 at P4–5) (from El Fata et al., 2014)
- (F)
Left, Calcium traces for 5 representative L2/3 neurons in barrel cortex of unanesthetized WT mice and Fmr1−/− mice at P14-16 showing synchronous bursts of cell firing (dashed lines) in the Fmr1−/− mouse. Right, Mean correlation coefficients for all cell pairs within 100 µm of each other for WT and Fmr1−/− mice at different postnatal ages. Both age and genotype significantly affected correlation coefficients,*p < 0.05). [from Goncalves et al., 2013]

- (A)
Left, Images of the vasculature through the cranial window (top) and intrinsic signal images (bottom) collected from a WT and a Fmr1 KO mouse after single whisker stimulation. Rostral (R), Caudal (C), Lateral (L) and Medial (M). Right, The region of response with DR/R magnitudes greater than the threshold is larger for Fmr1 KO than for WT mice (n= 10 each; WT vs. KO, p= 0.011). [from Arnett et al., 2014]
- (B)
Left, Five consecutive somatic responses to contralateral hindpaw stimulation (2 ms, 30 mA) recorded from L2/3 pyramidal neurons of S1 in anesthetized WT and Fmr1−/y littermate mice (APs indicated by arrows, top). Right, The average number of APs per trial of hindpaw stimulation was increased in neurons from Fmr1−/y mice (p < 0.05). [from Zhang et al., 2014]
- (C)
Left, Schematic of the gap-crossing apparatus (left). Successful localization of the object and gap-crossing was rewarded appetitively. For short distances, mice use their noses, whereas for long gaps (>4.5 cm) they use whiskers. Right, Percent improvement from first 6 sessions to last 6 sessions for short (nose) and long distances (whiskers). WT mice display significantly greater improvement at whisker-dependent distances than Fmr1 KO mice (p= 0.02) [from Arnett et al., 2014]

References
-
- Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. - PubMed
-
- Ayres AJ. Tactile Functions. Their Relation to Hyperactive and Perceptual Motor Behavior. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1964;18:6–11. - PubMed
-
- Baranek GT, Foster LG, Berkson G. Tactile defensiveness and stereotyped behaviors. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1997;51:91–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

