Regulatory Logic of Pan-Neuronal Gene Expression in C. elegans
- PMID: 26291158
- PMCID: PMC4545498
- DOI: 10.1016/j.neuron.2015.07.031
Regulatory Logic of Pan-Neuronal Gene Expression in C. elegans
Abstract
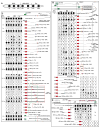
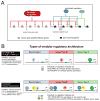
While neuronal cell types display an astounding degree of phenotypic diversity, most if not all neuron types share a core panel of terminal features. However, little is known about how pan-neuronal expression patterns are genetically programmed. Through an extensive analysis of the cis-regulatory control regions of a battery of pan-neuronal C. elegans genes, including genes involved in synaptic vesicle biology and neuropeptide signaling, we define a common organizational principle in the regulation of pan-neuronal genes in the form of a surprisingly complex array of seemingly redundant, parallel-acting cis-regulatory modules that direct expression to broad, overlapping domains throughout the nervous system. These parallel-acting cis-regulatory modules are responsive to a multitude of distinct trans-acting factors. Neuronal gene expression programs therefore fall into two fundamentally distinct classes. Neuron-type-specific genes are generally controlled by discrete and non-redundantly acting regulatory inputs, while pan-neuronal gene expression is controlled by diverse, coincident and seemingly redundant regulatory inputs.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures






References
-
- Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 2001;128:1951–1969. - PubMed
-
- Aoki H, Hara A, Era T, Kunisada T, Yamada Y. Genetic ablation of Rest leads to in vitro-specific derepression of neuronal genes during neurogenesis. Development. 2012;139:667–677. - PubMed
-
- Chen ZF, Paquette AJ, Anderson DJ. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet. 1998;20:136–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

