A Temporary Gating of Actin Remodeling during Synaptic Plasticity Consists of the Interplay between the Kinase and Structural Functions of CaMKII
- PMID: 26291163
- PMCID: PMC4548268
- DOI: 10.1016/j.neuron.2015.07.023
A Temporary Gating of Actin Remodeling during Synaptic Plasticity Consists of the Interplay between the Kinase and Structural Functions of CaMKII
Erratum in
- Neuron. 2015 Oct 21;88(2):433
Abstract
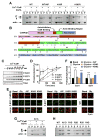
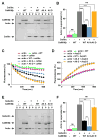
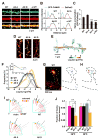
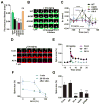
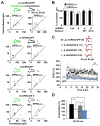
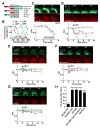
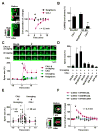
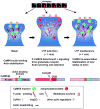
The structural modification of dendritic spines plays a critical role in synaptic plasticity. CaMKII is a pivotal molecule involved in this process through both kinase-dependent and independent structural functions, but the respective contributions of these two functions to the synaptic plasticity remain unclear. We demonstrate that the transient interplay between the kinase and structural functions of CaMKII during the induction of synaptic plasticity temporally gates the activity-dependent modification of the actin cytoskeleton. Inactive CaMKII binds F-actin, thereby limiting access of actin-regulating proteins to F-actin and stabilizing spine structure. CaMKII-activating stimuli trigger dissociation of CaMKII from F-actin through specific autophosphorylation reactions within the F-actin binding region and permits F-actin remodeling by regulatory proteins followed by reassociation and restabilization. Blocking the autophosphorylation impairs both functional and structural plasticity without affecting kinase activity. These results underpin the importance of the interplay between the kinase and structural functions of CaMKII in defining a time window permissive for synaptic plasticity.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement:
YH is partly supported by Takeda Pharmaceutical Co. Ltd. and Fujitsu Laboratories.
Figures
References
-
- Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem. 1999;274:22713–22722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

