Hepcidin and Host Defense against Infectious Diseases
- PMID: 26291319
- PMCID: PMC4546197
- DOI: 10.1371/journal.ppat.1004998
Hepcidin and Host Defense against Infectious Diseases
Abstract
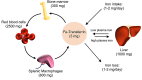
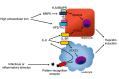
Hepcidin is the master regulator of iron homeostasis in vertebrates. The synthesis of hepcidin is induced by systemic iron levels and by inflammatory stimuli. While the role of hepcidin in iron regulation is well established, its contribution to host defense is emerging as complex and multifaceted. In this review, we summarize the literature on the role of hepcidin as a mediator of antimicrobial immunity. Hepcidin induction during infection causes depletion of extracellular iron, which is thought to be a general defense mechanism against many infections by withholding iron from invading pathogens. Conversely, by promoting iron sequestration in macrophages, hepcidin may be detrimental to cellular defense against certain intracellular infections, although critical in vivo studies are needed to confirm this concept. It is not yet clear whether hepcidin exerts any iron-independent effects on host defenses.
Conflict of interest statement
Michels and Mehrad have declared that no competing interests exist. We have read the journal's policy and have the following conflicts: Ganz and Nemeth are shareholders and consultants for Merganser Biotech, a company engaged in the development of minihepcidins that could be used to treat infections, and founders of Intrinsic Lifesciences, a company developing hepcidin-based diagnostics. This does not alter our adherence to all PLOS policies.
Figures

References
-
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing Acts: Molecular Control of Mammalian Iron Metabolism. Cell. 2004;117(3):285–97. - PubMed
-
- Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annual reviews in microbiology. 2000;54(1):881–941. - PubMed
-
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. Journal of biological chemistry. 2001;276(11):7806–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

