Monitoring Effect of Human Papillomavirus Vaccines in US Population, Emerging Infections Program, 2008-2012
- PMID: 26291379
- PMCID: PMC4550135
- DOI: 10.3201/eid2109.141841
Monitoring Effect of Human Papillomavirus Vaccines in US Population, Emerging Infections Program, 2008-2012
Abstract
In 2007, five Emerging Infections Program (EIP) sites were funded to determine the feasibility of establishing a population-based surveillance system for monitoring the effect of human papillomavirus (HPV) vaccine on pre-invasive cervical lesions. The project involved active population-based surveillance of cervical intraepithelial neoplasia grades 2 and 3 and adenocarcinoma in situ as well as associated HPV types in women >18 years of age residing in defined catchment areas; collecting relevant clinical information and detailed HPV vaccination histories for women 18-39 years of age; and estimating the annual rate of cervical cancer screening among the catchment area population. The first few years of the project provided key information, including data on HPV type distribution, before expected effect of vaccine introduction. The project's success exemplifies the flexibility of EIP's network to expand core activities to include emerging surveillance needs beyond acute infectious diseases. Project results contribute key information regarding the impact of HPV vaccination in the United States.
Keywords: CIN; CIN2; CIN3; Emerging Infections Program; HPV; HPV vaccine; United States; adenocarcinoma in situ; cancer; cervical intraepithelial neoplasia; cervical precancer; human papillomavirus; vaccination; vaccine effect; viruses.
Figures
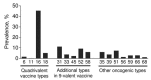
References
-
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1–30 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

