Which Way In? The RalF Arf-GEF Orchestrates Rickettsia Host Cell Invasion
- PMID: 26291822
- PMCID: PMC4546372
- DOI: 10.1371/journal.ppat.1005115
Which Way In? The RalF Arf-GEF Orchestrates Rickettsia Host Cell Invasion
Abstract
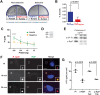
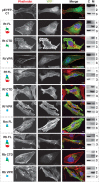
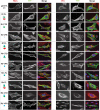
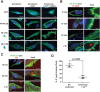
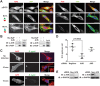
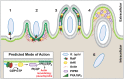
Bacterial Sec7-domain-containing proteins (RalF) are known only from species of Legionella and Rickettsia, which have facultative and obligate intracellular lifestyles, respectively. L. pneumophila RalF, a type IV secretion system (T4SS) effector, is a guanine nucleotide exchange factor (GEF) of ADP-ribosylation factors (Arfs), activating and recruiting host Arf1 to the Legionella-containing vacuole. In contrast, previous in vitro studies showed R. prowazekii (Typhus Group) RalF is a functional Arf-GEF that localizes to the host plasma membrane and interacts with the actin cytoskeleton via a unique C-terminal domain. As RalF is differentially encoded across Rickettsia species (e.g., pseudogenized in all Spotted Fever Group species), it may function in lineage-specific biology and pathogenicity. Herein, we demonstrate RalF of R. typhi (Typhus Group) interacts with the Rickettsia T4SS coupling protein (RvhD4) via its proximal C-terminal sequence. RalF is expressed early during infection, with its inactivation via antibody blocking significantly reducing R. typhi host cell invasion. For R. typhi and R. felis (Transitional Group), RalF ectopic expression revealed subcellular localization with the host plasma membrane and actin cytoskeleton. Remarkably, R. bellii (Ancestral Group) RalF showed perinuclear localization reminiscent of ectopically expressed Legionella RalF, for which it shares several structural features. For R. typhi, RalF co-localization with Arf6 and PI(4,5)P2 at entry foci on the host plasma membrane was determined to be critical for invasion. Thus, we propose recruitment of PI(4,5)P2 at entry foci, mediated by RalF activation of Arf6, initiates actin remodeling and ultimately facilitates bacterial invasion. Collectively, our characterization of RalF as an invasin suggests that, despite carrying a similar Arf-GEF unknown from other bacteria, different intracellular lifestyles across Rickettsia and Legionella species have driven divergent roles for RalF during infection. Furthermore, our identification of lineage-specific Arf-GEF utilization across some rickettsial species illustrates different pathogenicity factors that define diverse agents of rickettsial diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Garcia-del Portillo F, Finlay BB. The varied lifestyles of intracellular pathogens within eukaryotic vacuolar compartments. Trends Microbiol. 1995;3: 373–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

