Controlled Human Malaria Infection (CHMI) differentially affects cell-mediated and antibody responses to CSP and AMA1 induced by adenovirus vaccines with and without DNA-priming
- PMID: 26292027
- PMCID: PMC4685686
- DOI: 10.1080/21645515.2015.1019186
Controlled Human Malaria Infection (CHMI) differentially affects cell-mediated and antibody responses to CSP and AMA1 induced by adenovirus vaccines with and without DNA-priming
Abstract
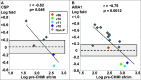
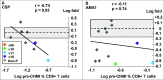
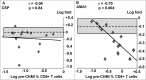
We have previously shown that a DNA-prime followed by an adenovirus-5 boost vaccine containing CSP and AMA1 (DNA/Ad) successfully protected 4 of 15 subjects to controlled human malaria infection (CHMI). However, the adenovirus-5 vaccine alone (AdCA) failed to induce protection despite eliciting cellular responses that were often higher than those induced by DNA/Ad. Here we determined the effect of CHMI on pre-CHMI cellular and antibody responses against CSP and AMA1 expressed as fold-changes in activities. Generally, in the DNA/Ad trial, CHMI caused pre-CHMI ELISpot IFN-γ and CD8+ T cell IFN-γ responses of the protected subjects to fall but among non-protected subjects, CHMI caused rises of pre-CHMI ELISpot IFN-γ but falls of CD8+ T cell IFN-γ responses. In contrast in the AdCA trial, CHMI caused both pre-CHMI ELISpot IFN-γ and CD8+ T cell IFN-γ responses of the AdCA subjects to fall. We suggest that the falls in activities are due to migration of peripheral CD8+ T cells to the liver in response to developing liver stage parasites, and this fall, in the DNA/Ad trial, is masked in ELISpot responses of the non-protected subjects by rises in other immune cell types. In addition, CHMI caused falls in antibody activities of protected subjects, but rises in non-protected subjects in both trials to CSP, and dramatically in the AdCA trial to AMA1, reaching 380 μg/ml that is probably due to boosting by transient blood stage infection before chloroquine treatment. Taken together, these results further define differences in cellular responses between DNA/Ad and AdCA trials, and suggest that natural transmission may boost responses induced by these malaria vaccines especially when protection is not achieved.
Trial registration: ClinicalTrials.gov NCT00392015 NCT00870987.
Keywords: AMA1; CHMI; CSP; DNA -prime; T cells; adenovirus-boost; antibody; efficacy; malaria; vaccine.
Figures



References
-
- Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, Patterson N, Guerrero M, Bennett JW, McGrath MG, et al.. DNA prime/adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One 2013; 8:1371; PMID:23457473; http://dx.doi.org/10.1371/journal.pone.0055571 - DOI - PMC - PubMed
-
- Tamminga C, Sedegah M, Maiolatesi S, Fedders C, Reyes S, Reyes A, Vasquez C, Alcorta Y, Chuang I, Spring M, et al.. Human adenovirus 5-vectored Plasmodium falciparum NMRC-M3V-Ad-PfCA vaccine encoding CSP and AMA1 is safe, well-tolerated and immunogenic but does not protect against controlled human malaria infection. Hum Vaccin Immunother 2013; 9:2165-2177; PMID:23899517; http://dx.doi.org/10.4161/hv.24941 - DOI - PMC - PubMed
-
- Sedegah M, Hollingdale MR, Farooq F, Ganeshan H, Belmonte M, Kim Y, Peters B, Sette A, Huang J, McGrath S, et al.. Sterile Immunity to Malaria after DNA Prime/Adenovirus Boost Immunization Is Associated with Effector Memory CD8+T Cells Targeting AMA1 Class I Epitopes. PLoS One 2014; 9:e106241; PMID:25211344; http://dx.doi.org/10.1371/journal.pone.0106241 - DOI - PMC - PubMed
-
- Zarling S, Berenzon D, Dalai S, Liepinsh D, Steers N, Krzych U. The survival of memory CD8 T cells that is mediated by IL-15 correlates with sustained protection against malaria. J Immunol 2013; 190:5128-5141; PMID:23589611; http://dx.doi.org/10.4049/jimmunol.1203396 - DOI - PMC - PubMed
-
- Nganou-Makamdop K, van Gemert GJ, Arens T, Hermsen CC, Sauerwein RW. Long term protection after immunization with P. berghei sporozoites correlates with sustained IFNgamma responses of hepatic CD8+ memory T cells. PLoS One 2012; 7:e36508; PMID:22563506; http://dx.doi.org/10.1371/journal.pone.0036508 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
