Discovery of Novel Plasmodium falciparum Pre-Erythrocytic Antigens for Vaccine Development
- PMID: 26292257
- PMCID: PMC4546230
- DOI: 10.1371/journal.pone.0136109
Discovery of Novel Plasmodium falciparum Pre-Erythrocytic Antigens for Vaccine Development
Abstract
Background: Nearly 100% protection against malaria infection can be achieved in humans by immunization with P. falciparum radiation-attenuated sporozoites (RAS). Although it is thought that protection is mediated by T cell and antibody responses, only a few of the many pre-erythrocytic (sporozoite and liver stage) antigens that are targeted by these responses have been identified.
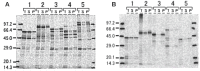
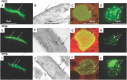
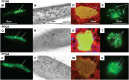
Methodology: Twenty seven P. falciparum pre-erythrocytic antigens were selected using bioinformatics analysis and expression databases and were expressed in a wheat germ cell-free protein expression system. Recombinant proteins were recognized by plasma from RAS-immunized subjects, and 21 induced detectable antibody responses in mice and rabbit and sera from these immunized animals were used to characterize these antigens. All 21 proteins localized to the sporozoite: five localized to the surface, seven localized to the micronemes, cytoplasm, endoplasmic reticulum or nucleus, two localized to the surface and cytoplasm, and seven remain undetermined. PBMC from RAS-immunized volunteers elicited positive ex vivo or cultured ELISpot responses against peptides from 20 of the 21 antigens.
Conclusions: These T cell and antibody responses support our approach of using reagents from RAS-immunized subjects to screen potential vaccine antigens, and have led to the identification of a panel of novel P. falciparum antigens. These results provide evidence to further evaluate these antigens as vaccine candidates.
Trial registration: ClinicalTrials.gov NCT00870987 ClinicalTrials.gov NCT00392015.
Conflict of interest statement
Figures



References
-
- Weiss WR, Good MF, Hollingdale MR, Miller LH, Berzofsky JA. Genetic control of immunity to Plasmodium yoelii sporozoites. J Immunol. 1989;143(12):4263–6. - PubMed
-
- Nussenzweig V, Nussenzweig RS. Circumsporozoite proteins of malaria parasites. Cell. 1985;42(2):401–3. - PubMed
-
- Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24(3):397–401. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

