Compassionate use of orphan drugs
- PMID: 26292942
- PMCID: PMC4546220
- DOI: 10.1186/s13023-015-0306-x
Compassionate use of orphan drugs
Abstract
Background: EU regulation 726/2004 authorises manufacturers to provide drugs to patients on a temporary basis when marketing authorisation sought centrally for the entire EU is still pending. Individual Member States retain the right to approve and implement such 'compassionate use' programmes which companies will usually provide for free. Nevertheless some companies have opted not to partake in such programmes, in effect restricting access to drugs for patients in need. Here we survey the state of compassionate use programmes in the EU with particular reference to the rare disease field, and provide legal and ethical arguments to encourage their increased compassionate use in the EU and beyond. We contend that if enacted, these recommendations will be mutually beneficial to companies as well as patients.
Methods: Requests for information from the European Medicines Agency were made under the UK Freedom of Information Act 2000. Legal, ethical and economic/pragmatic analysis identified means by which provision of therapy in compassionate use programmes might be increased.
Results: More than 50 notifications of compassionate use programmes have been submitted to the EMA by Member States since 2006. About 40 % relate to orphan drugs. As there is a compulsory register of programmes but not of outcomes, their success is difficult to evaluate but, for example, the French programme expedited treatment for more than 20,000 (orphan and non-orphan) patients over a period of three years.
Conclusion: Compelling self-interested, legal and ethical arguments can be mounted to encourage manufacturers to offer therapies on a compassionate use basis and these are often equally applicable to provision on a humanitarian aid basis. The EU's compassionate use programmes are instrumental in ensuring continuity of access to drugs until approval and reimbursement decisions are finalised. We propose the creation of a registry of drugs offered on a compassionate use basis; further transparency would allow such programmes to be evaluated and direct patients to sources of treatment.
Figures
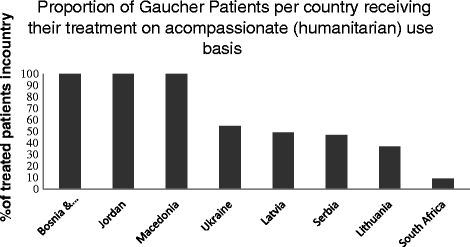
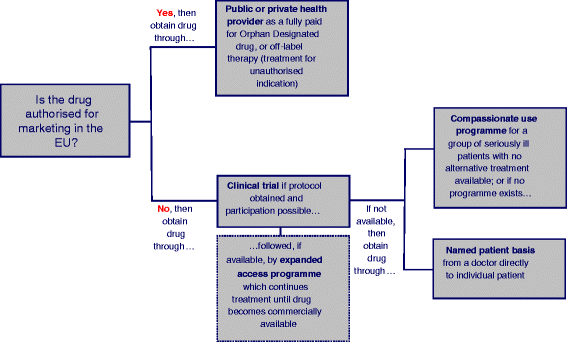
References
-
- European Medicines Agency. Pilot project on adaptive licensing. 19 March 2014. [http://www.ema.europa.eu/docs/en_GB/document_library/Other/2014/03/WC500...]
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

