Parainflammation, chronic inflammation, and age-related macular degeneration
- PMID: 26292978
- PMCID: PMC4733662
- DOI: 10.1189/jlb.3RI0615-239R
Parainflammation, chronic inflammation, and age-related macular degeneration
Abstract
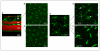
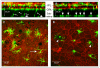
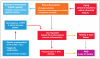
Inflammation is an adaptive response of the immune system to noxious insults to maintain homeostasis and restore functionality. The retina is considered an immune-privileged tissue as a result of its unique anatomic and physiologic properties. During aging, the retina suffers from a low-grade chronic oxidative insult, which sustains for decades and increases in level with advancing age. As a result, the retinal innate-immune system, particularly microglia and the complement system, undergoes low levels of activation (parainflammation). In many cases, this parainflammatory response can maintain homeostasis in the healthy aging eye. However, in patients with age-related macular degeneration, this parainflammatory response becomes dysregulated and contributes to macular damage. Factors contributing to the dysregulation of age-related retinal parainflammation include genetic predisposition, environmental risk factors, and old age. Dysregulated parainflammation (chronic inflammation) in age-related macular degeneration damages the blood retina barrier, resulting in the breach of retinal-immune privilege, leading to the development of retinal lesions. This review discusses the basic principles of retinal innate-immune responses to endogenous chronic insults in normal aging and in age-related macular degeneration and explores the difference between beneficial parainflammation and the detrimental chronic inflammation in the context of age-related macular degeneration.
Keywords: blood-retina barrier; complement; immune privilege; microglia; retina.
© Society for Leukocyte Biology.
Figures

References
-
- Mangge H, Almer G, Truschnig-Wilders M, Schmidt A, Gasser R, Fuchs D. Inflammation, adiponectin, obesity and cardiovascular risk. Curr.Med.Chem. 2010;17:4511–4520. - PubMed
-
- Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin.Pharmacol.Ther. 2010;87:407–416. - PubMed
-
- Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–1932. - PubMed
-
- McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann.N.Y.Acad.Sci. 2004;1035:104–116. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

