Control of vertebrate core planar cell polarity protein localization and dynamics by Prickle 2
- PMID: 26293301
- PMCID: PMC4631750
- DOI: 10.1242/dev.121384
Control of vertebrate core planar cell polarity protein localization and dynamics by Prickle 2
Abstract
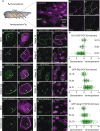
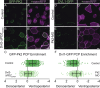
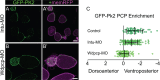
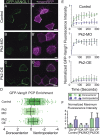
Planar cell polarity (PCP) is a ubiquitous property of animal tissues and is essential for morphogenesis and homeostasis. In most cases, this fundamental property is governed by a deeply conserved set of 'core PCP' proteins, which includes the transmembrane proteins Van Gogh-like (Vangl) and Frizzled (Fzd), as well as the cytoplasmic effectors Prickle (Pk) and Dishevelled (Dvl). Asymmetric localization of these proteins is thought to be central to their function, and understanding the dynamics of these proteins is an important challenge in developmental biology. Among the processes that are organized by the core PCP proteins is the directional beating of cilia, such as those in the vertebrate node, airway and brain. Here, we exploit the live imaging capabilities of Xenopus to chart the progressive asymmetric localization of fluorescent reporters of Dvl1, Pk2 and Vangl1 in a planar polarized ciliated epithelium. Using this system, we also characterize the influence of Pk2 on the asymmetric dynamics of Vangl1 at the cell cortex, and we define regions of Pk2 that control its own localization and those impacting Vangl1. Finally, our data reveal a striking uncoupling of Vangl1 and Dvl1 asymmetry. This study advances our understanding of conserved PCP protein functions and also establishes a rapid, tractable platform to facilitate future in vivo studies of vertebrate PCP protein dynamics.
Keywords: Cilia; Core PCP; Dishevelled; Dvl1; Multiciliated cell; Pk2; Planar cell polarity; Prickle; Van Gogh-like; Vangl1; Xenopus.
© 2015. Published by The Company of Biologists Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

