Monocyte-Derived Dendritic Cells Upregulate Extracellular Catabolism of Aggregated Low-Density Lipoprotein on Maturation, Leading to Foam Cell Formation
- PMID: 26293468
- PMCID: PMC4583358
- DOI: 10.1161/ATVBAHA.115.305843
Monocyte-Derived Dendritic Cells Upregulate Extracellular Catabolism of Aggregated Low-Density Lipoprotein on Maturation, Leading to Foam Cell Formation
Abstract
Objective: Although dendritic cells are known to play a role in atherosclerosis, few studies have examined the contribution of the wide variety of dendritic cell subsets. Accordingly, their roles in atherogenesis remain largely unknown. We investigated the ability of different dendritic cell subsets to become foam cells after contact with aggregated low-density lipoprotein (LDL; the predominant form of LDL found in atherosclerotic plaques).
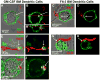
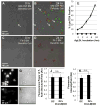
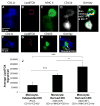
Approach and results: We demonstrate that both murine and human monocyte-derived dendritic cells use exophagy to degrade aggregated LDL, leading to foam cell formation, whereas monocyte-independent dendritic cells are unable to clear LDL aggregates by this mechanism. Exophagy is a catabolic process in which objects that cannot be internalized by phagocytosis (because of their size or association with extracellular structures) are initially digested in an extracellular acidic lytic compartment. Surprisingly, we found that monocyte-derived dendritic cells upregulate exophagy on maturation. This contrasts various forms of endocytic internalization in dendritic cells, which decrease on maturation. Finally, we show that our in vitro results are consistent with dendritic cell lipid accumulation in plaques of an ApoE(-/-) mouse model of atherosclerosis.
Conclusions: Our results show that monocyte-derived dendritic cells use exophagy to degrade aggregated LDL and become foam cells, whereas monocyte-independent dendritic cells are unable to clear LDL deposits. Furthermore, we find that exophagy is upregulated on dendritic cell maturation. Thus, exophagy-mediated foam cell formation in monocyte-derived dendritic cells could play a significant role in atherogenesis.
Keywords: LDL; atherosclerosis; dendritic cells; foam cells; lipoproteins; phagocytosis.
© 2015 American Heart Association, Inc.
Figures



References
-
- Bobryshev YV, Lord RS. Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of Vascular Dendritic Cells in athero-resistant and athero-prone areas of the normal aorta. Arch Histol Cytol. 1995;58:307–322. - PubMed
-
- Bobryshev YV, Lord RS. Structural heterogeneity and contacting interactions of vascular dendritic cells in early atherosclerotic lesions of the human aorta. J Submicrosc Cytol Pathol. 1996;28:49–60. - PubMed
-
- Bobryshev YV. Dendritic cells and their involvement in atherosclerosis. Current Opinion in Lipidology. 2000;11:511–517. - PubMed
-
- Bobryshev YV, Lord RS. S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovascular Research. 1995;29:689–696. - PubMed
-
- Bobryshev YV, Ikezawa T, Watanabe T. Formation of Birbeck granule-like structures in vascular dendritic cells in human atherosclerotic aorta. Lag-antibody to epidermal Langerhans cells recognizes cells in the aortic wall. Atherosclerosis. 1997;133:193–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

