Foxp3-modified bone marrow mesenchymal stem cells promotes liver allograft tolerance through the generation of regulatory T cells in rats
- PMID: 26293578
- PMCID: PMC4545923
- DOI: 10.1186/s12967-015-0638-2
Foxp3-modified bone marrow mesenchymal stem cells promotes liver allograft tolerance through the generation of regulatory T cells in rats
Abstract
Background: The transcription factor forkhead box P3 (Foxp3) is a master regulatory gene necessary for the development and function of CD4(+)CD25(+) regulatory T cells (Tregs). Mesenchymal stem cells (MSC) have recently emerged as promising candidates for cell-based immunosuppression/tolerance induction protocols. Thus, we hypothesized that MSC-based Foxp3 gene therapy would improve immunosuppressive capacity of MSC and induce donor-specific allograft tolerance in rat's liver allograft model.
Methods: The present study utilized a lentivirus vector to overexpress the therapeutic gene Foxp3 on MSC. In vivo, Injections of 2 × 10(6) MSC, FUGW-MSC or Foxp3-MSC into the portal vein were carried out immediately after liver transplantation.
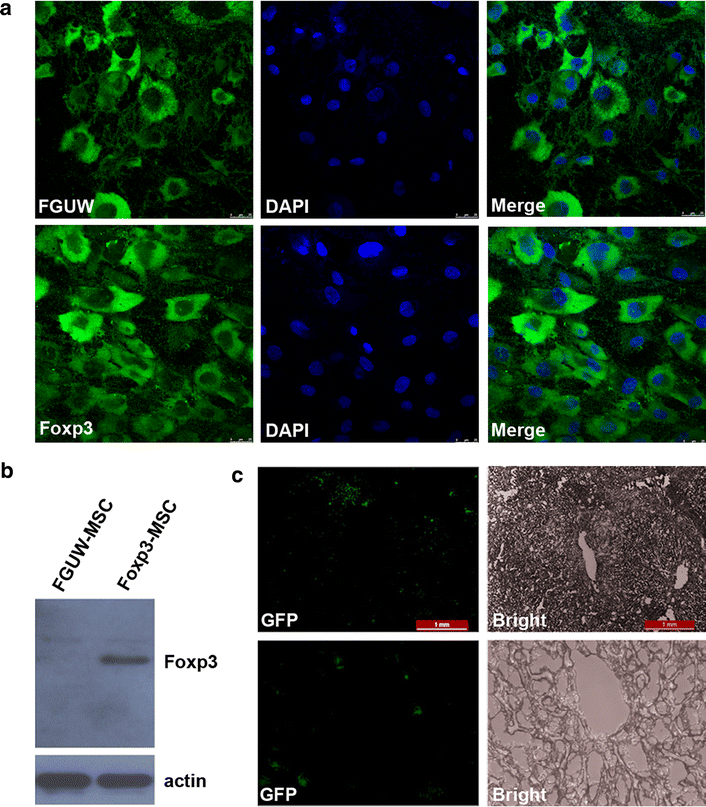
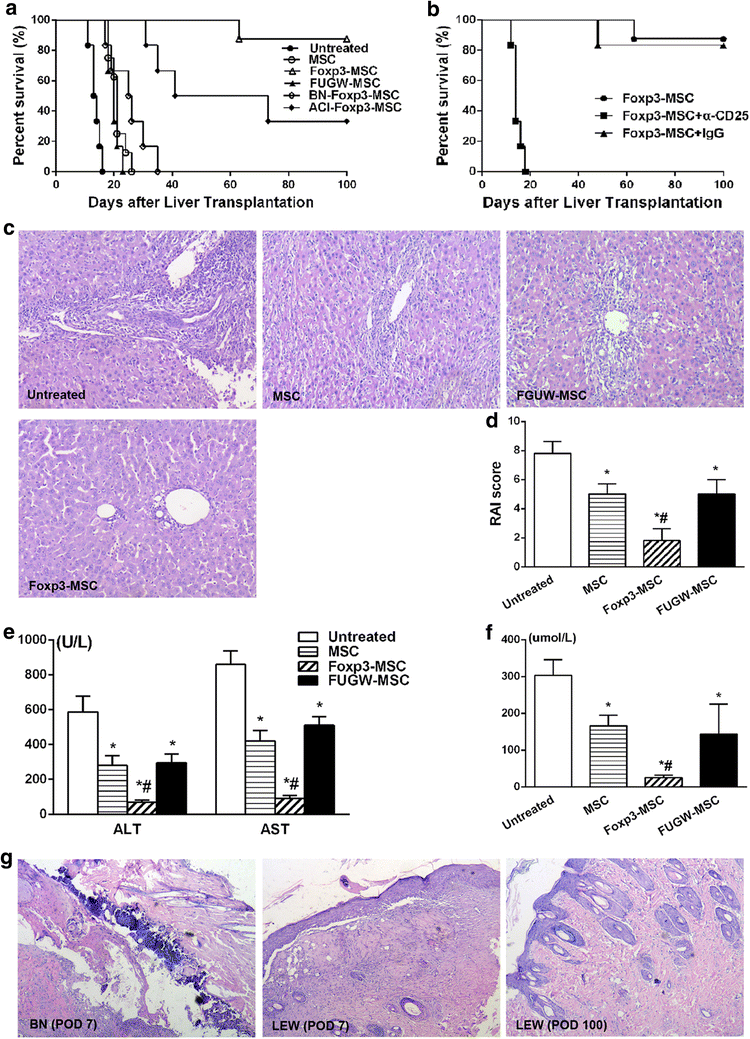
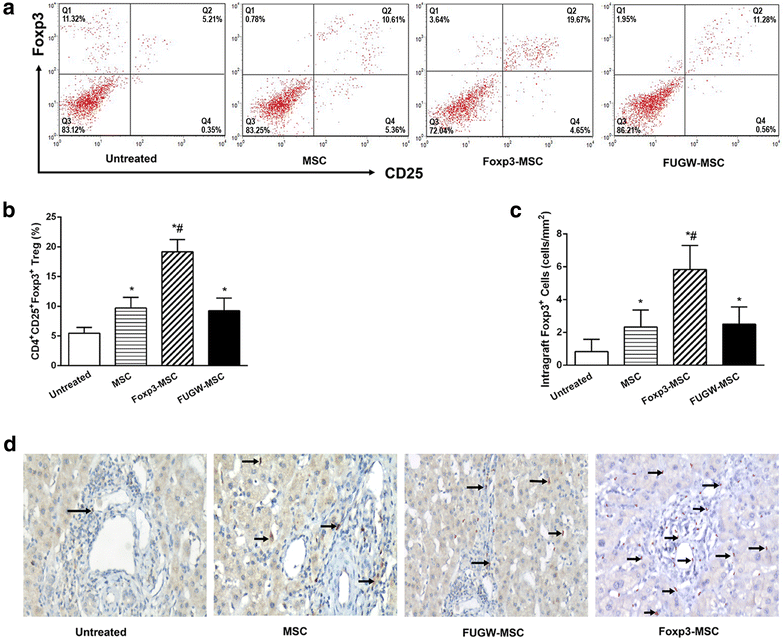
Results: Successful gene transfer of Foxp3 in MSC was achieved by lentivirus carrying Foxp3 and Foxp3-MSC engraftment in liver allograft was confirmed by fluorescence microscopy. Foxp3-MSC treatment significantly inhibited the proliferation of allogeneic ACI CD4(+) T cells to splenocytes (SC) from the same donor strain or third-party BN rat compared with MSC. Foxp3-MSC suppressive effect on the proliferation of CD4(+) T cells is contact dependent and associated with Programmed death ligand 1(PD-L1) upregulation in MSC. Co-culture of CD4(+) T cells with Foxp3-MSC results in a shift towards a Tregs phenotype. More importantly, Foxp3-MSC monotherapy achieved donor-specific liver allograft tolerance and generated a state of CD4(+)CD25(+)Foxp3(+) Tregs-dependent tolerance.
Conclusion: Foxp3-engineered MSC therapy seems to be a promising and attractive cell therapy approach for inducing immunosuppression or transplant tolerance.
Figures

References
-
- Long E, Wood KJ. Understanding FOXP3: progress towards achieving transplantation tolerance. Transplantation. 2007;84(4):459–461. doi: 10.1097/01.tp.0000275424.52998.ad. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

