Serum Biomarkers of Inflammation, Fibrosis, and Cardiac Function in Facilitating Diagnosis, Prognosis, and Treatment of Anti-SSA/Ro-Associated Cardiac Neonatal Lupus
- PMID: 26293764
- PMCID: PMC4545752
- DOI: 10.1016/j.jacc.2015.06.1088
Serum Biomarkers of Inflammation, Fibrosis, and Cardiac Function in Facilitating Diagnosis, Prognosis, and Treatment of Anti-SSA/Ro-Associated Cardiac Neonatal Lupus
Erratum in
- J Am Coll Cardiol. 2015 Oct 20;66(16):1849
Abstract
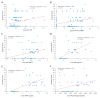
Background: Cardiac manifestations of neonatal lupus (cardiac NL) include congenital heart block and cardiomyopathy. Several candidate biomarkers were evaluated in cases at risk for cardiac NL on the basis of potential roles in inflammation, fibrosis, and cardiac dysfunction: C-reactive protein (CRP); NT-pro-B-type natriuretic peptide (NT-proBNP); troponin I; matrix metalloproteinase (MMP)-2; urokinase plasminogen activator (uPA); urokinase plasminogen activator receptor (uPAR); plasminogen; and vitamin D.
Objectives: Identification of maternal and fetal biomarkers associated with development and morbidity of cardiac NL should provide clues to pathogenesis with translational implications for management.
Methods: Cord (139) and maternal (135) blood samples collected during pregnancies at risk for cardiac NL were available for study. Levels of cord and maternal CRP, cord NT-proBNP, and cord troponin I were evaluated using multiplex assays. Cord and maternal vitamin D were assessed by liquid chromatography-mass spectrometry. MMP-2, uPA, uPAR, and plasminogen were evaluated using ELISA.
Results: Cord CRP, NT-proBNP, MMP-2, uPA, uPAR, and plasminogen levels were higher in cardiac NL-affected fetuses than in unaffected cases, independent of maternal rheumatic disease, season at highest risk of cardiac NL development, and medications taken during pregnancy. These biomarkers were positively associated with a disease severity score derived from known risk factors for mortality in cardiac NL. Maternal CRP and cord troponin I levels did not differ between the groups. Cord and maternal vitamin D levels were not significantly associated with cardiac NL, but average maternal vitamin D level during pregnancy was positively associated with longer time to postnatal pacemaker placement.
Conclusions: These data support the association of fetal reactive inflammatory and fibrotic components with development and morbidity of cardiac NL. Following CRP and NT-proBNP levels after birth can potentially monitor severity and progression of cardiac NL. MMP-2 and the uPA/uPAR/plasminogen cascade provide therapeutic targets to decrease fibrosis. Although decreased vitamin D did not confer increased risk, given the positive influence on postnatal outcomes, maternal levels should be optimized.
Keywords: antibody; congenital heart disease; heart block.
Copyright © 2015 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Cord Blood Samples: A Less Explored Tool in Early Diagnosis of Neonatal Cardiovascular Disease.J Am Coll Cardiol. 2015 Aug 25;66(8):940-2. doi: 10.1016/j.jacc.2015.07.001. J Am Coll Cardiol. 2015. PMID: 26293765 No abstract available.
Similar articles
-
A central role of plasmin in cardiac injury initiated by fetal exposure to maternal anti-Ro autoantibodies.Rheumatology (Oxford). 2013 Aug;52(8):1448-53. doi: 10.1093/rheumatology/ket156. Epub 2013 Apr 18. Rheumatology (Oxford). 2013. PMID: 23598443 Free PMC article.
-
Umbilical cord blood levels of maternal antibodies reactive with p200 and full-length Ro 52 in the assessment of risk for cardiac manifestations of neonatal lupus.Arthritis Care Res (Hoboken). 2012 Sep;64(9):1373-81. doi: 10.1002/acr.21704. Arthritis Care Res (Hoboken). 2012. PMID: 22511615 Free PMC article.
-
Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine.Ann Rheum Dis. 2010 Oct;69(10):1827-30. doi: 10.1136/ard.2009.119263. Epub 2010 May 6. Ann Rheum Dis. 2010. PMID: 20447951 Free PMC article.
-
[Neonatal lupus erythematosus].Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(2):124-130. doi: 10.2177/jsci.40.124. Nihon Rinsho Meneki Gakkai Kaishi. 2017. PMID: 28603203 Review. Japanese.
-
Neonatal lupus: advances in understanding pathogenesis and identifying treatments of cardiac disease.Curr Opin Rheumatol. 2012 Sep;24(5):466-72. doi: 10.1097/BOR.0b013e328356226b. Curr Opin Rheumatol. 2012. PMID: 22832822 Free PMC article. Review.
Cited by
-
Molecular Mechanisms of Fetal and Neonatal Lupus: A Narrative Review of an Autoimmune Disease Transferal across the Placenta.Int J Mol Sci. 2024 May 10;25(10):5224. doi: 10.3390/ijms25105224. Int J Mol Sci. 2024. PMID: 38791261 Free PMC article. Review.
-
GPLD1 Attenuates Heart Failure via Dual-Membrane Localization to Inhibit uPAR.Circ Res. 2025 Aug 15;137(5):e124-e143. doi: 10.1161/CIRCRESAHA.124.325623. Epub 2025 Jul 9. Circ Res. 2025. PMID: 40631685
-
Siglec-1 Macrophages and the Contribution of IFN to the Development of Autoimmune Congenital Heart Block.J Immunol. 2019 Jan 1;202(1):48-55. doi: 10.4049/jimmunol.1800357. Epub 2018 Dec 5. J Immunol. 2019. PMID: 30518570 Free PMC article.
-
The prevention, screening and treatment of congenital heart block from neonatal lupus: a survey of provider practices.Rheumatology (Oxford). 2018 Jul 1;57(suppl_5):v9-v17. doi: 10.1093/rheumatology/key141. Rheumatology (Oxford). 2018. PMID: 30137589 Free PMC article.
-
Progress in the pathogenesis and treatment of cardiac manifestations of neonatal lupus.Curr Opin Rheumatol. 2017 Sep;29(5):467-472. doi: 10.1097/BOR.0000000000000414. Curr Opin Rheumatol. 2017. PMID: 28520682 Free PMC article. Review.
References
-
- Scott JS, Maddison PJ, Taylor PV, et al. Connective-tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983;309:209–12. - PubMed
-
- Buyon JP, Friedman DM. Neonatal lupus. In: Lahita RG, Tsokos G, Buyon JP, et al., editors. Systemic Lupus Erythematosus. 5. New York, NY: Academic Press; 2010. pp. 541–71.
-
- Clancy RM, Backer CB, Yin X, et al. Cytokine polymorphisms and histologic expression in autopsy studies: contribution of TNF-alpha and TGF-beta 1 to the pathogenesis of autoimmune-associated congenital heart block. J Immunol. 2003;171:3253–61. - PubMed
-
- Eliasson H, Sonesson SE, Sharland G, et al. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation. 2011;124:1919–26. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

