T Lymphocyte-Specific Activation of Nrf2 Protects from AKI
- PMID: 26293820
- PMCID: PMC4657838
- DOI: 10.1681/ASN.2014100978
T Lymphocyte-Specific Activation of Nrf2 Protects from AKI
Abstract
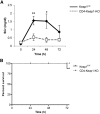
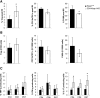
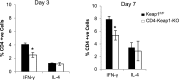
T lymphocytes are established mediators of ischemia reperfusion (IR)-induced AKI, but traditional immune principles do not explain their mechanism of early action in the absence of alloantigen. Nrf2 is a transcription factor that is crucial for cytoprotective gene expression and is generally thought to have a key role in dampening IR-induced AKI through protective effects on epithelial cells. We proposed an alternative hypothesis that augmentation of Nrf2 in T cells is essential to mitigate oxidative stress during IR-induced AKI. We therefore generated mice with genetically amplified levels of Nrf2 specifically in T cells and examined the effect on antioxidant gene expression, T cell activation, cytokine production, and IR-induced AKI. T cell-specific augmentation of Nrf2 significantly increased baseline antioxidant gene expression. These mice had a high frequency of intrarenal CD25(+)Foxp3(+) regulatory T cells and decreased frequencies of CD11b(+)CD11c(+) and F4/80(+) cells. Intracellular levels of TNF-α, IFN-γ, and IL-17 were significantly lower in CD4(+) T cells with high Nrf2 expression. Mice with increased T cell expression of Nrf2 were significantly protected from functional and histologic consequences of AKI. Furthermore, adoptive transfer of high-Nrf2 T cells protected wild-type mice from IR injury and significantly improved their survival. These data demonstrate that T cell-specific activation of Nrf2 protects from IR-induced AKI, revealing a novel mechanism of tissue protection during acute injury responses.
Keywords: AKI; Nrf2-Keap1; T cell; inflammation; oxidative stress.
Copyright © 2015 by the American Society of Nephrology.
Figures







Comment in
-
Oxidative Stress and Metabolism: The NF-Erythroid 2 p45-Related Factor 2:Kelch-like ECH-Associated Protein 1 System and Regulatory T Lymphocytes in Ischemic AKI.J Am Soc Nephrol. 2015 Dec;26(12):2893-5. doi: 10.1681/ASN.2015060720. Epub 2015 Aug 20. J Am Soc Nephrol. 2015. PMID: 26293819 Free PMC article. No abstract available.
References
-
- Clarkson MR, Friedewald JJ, Eustace JA, Rabb H: Acute kidney injury. In: Brenner & Rector’s The Kidney, edited by Brenner BM, 8th Ed., Philadelphia, Saunders, Elsevier, 2008, pp 943–986
-
- Srisawat N, Kellum JA: Acute kidney injury: Definition, epidemiology, and outcome. Curr Opin Crit Care 17: 548–555, 2011 - PubMed
-
- Rewa O, Bagshaw SM: Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014 - PubMed
-
- Jang HR, Ko GJ, Wasowska BA, Rabb H: The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med (Berl) 87: 859–864, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

