PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex
- PMID: 26293922
- PMCID: PMC4631654
- DOI: 10.1158/2159-8290.CD-15-0460
PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex
Abstract
mTOR serves as a central regulator of cell growth and metabolism by forming two distinct complexes, mTORC1 and mTORC2. Although mechanisms of mTORC1 activation by growth factors and amino acids have been extensively studied, the upstream regulatory mechanisms leading to mTORC2 activation remain largely elusive. Here, we report that the pleckstrin homology (PH) domain of SIN1, an essential and unique component of mTORC2, interacts with the mTOR kinase domain to suppress mTOR activity. More importantly, PtdIns(3,4,5)P3, but not other PtdInsPn species, interacts with SIN1-PH to release its inhibition on the mTOR kinase domain, thereby triggering mTORC2 activation. Mutating critical SIN1 residues that mediate PtdIns(3,4,5)P3 interaction inactivates mTORC2, whereas mTORC2 activity is pathologically increased by patient-derived mutations in the SIN1-PH domain, promoting cell growth and tumor formation. Together, our study unravels a PI3K-dependent mechanism for mTORC2 activation, allowing mTORC2 to activate AKT in a manner that is regulated temporally and spatially by PtdIns(3,4,5)P3.
Significance: The SIN1-PH domain interacts with the mTOR kinase domain to suppress mTOR activity, and PtdIns(3,4,5)P3 binds the SIN1-PH domain to release its inhibition on the mTOR kinase domain, leading to mTORC2 activation. Cancer patient-derived SIN1-PH domain mutations gain oncogenicity by loss of suppressing mTOR activity as a means to facilitate tumorigenesis.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures




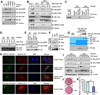

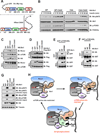
References
-
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. - PubMed
-
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. - PubMed
-
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

