Tipping the Scale from Disorder to Alpha-helix: Folding of Amphiphilic Peptides in the Presence of Macroscopic and Molecular Interfaces
- PMID: 26295346
- PMCID: PMC4546688
- DOI: 10.1371/journal.pcbi.1004328
Tipping the Scale from Disorder to Alpha-helix: Folding of Amphiphilic Peptides in the Presence of Macroscopic and Molecular Interfaces
Abstract
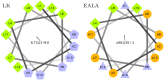
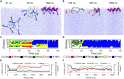
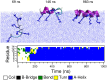
Secondary amphiphilicity is inherent to the secondary structural elements of proteins. By forming energetically favorable contacts with each other these amphiphilic building blocks give rise to the formation of a tertiary structure. Small proteins and peptides, on the other hand, are usually too short to form multiple structural elements and cannot stabilize them internally. Therefore, these molecules are often found to be structurally ambiguous up to the point of a large degree of intrinsic disorder in solution. Consequently, their conformational preference is particularly susceptible to environmental conditions such as pH, salts, or presence of interfaces. In this study we use molecular dynamics simulations to analyze the conformational behavior of two synthetic peptides, LKKLLKLLKKLLKL (LK) and EAALAEALAEALAE (EALA), with built-in secondary amphiphilicity upon forming an alpha-helix. We use these model peptides to systematically study their aggregation and the influence of macroscopic and molecular interfaces on their conformational preferences. We show that the peptides are neither random coils in bulk water nor fully formed alpha helices, but adopt multiple conformations and secondary structure elements with short lifetimes. These provide a basis for conformation-selection and population-shift upon environmental changes. Differences in these peptides' response to macroscopic and molecular interfaces (presented by an aggregation partner) can be linked to their inherent alpha-helical tendencies in bulk water. We find that the peptides' aggregation behavior is also strongly affected by presence or absence of an interface, and rather subtly depends on their surface charge and hydrophobicity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Molecular mechanism of β-sheet self-organization at water-hydrophobic interfaces.Proteins. 2011 Jan;79(1):1-22. doi: 10.1002/prot.22854. Epub 2010 Oct 11. Proteins. 2011. PMID: 20938982
-
Sequence and conformational preferences at termini of α-helices in membrane proteins: role of the helix environment.Proteins. 2014 Dec;82(12):3420-36. doi: 10.1002/prot.24696. Epub 2014 Oct 10. Proteins. 2014. PMID: 25257385
-
Environmental polarity induces conformational transitions in a helical peptide sequence from bacteriophage T4 lysozyme and its tandem duplicate: a molecular dynamics simulation study.J Mol Model. 2015 Apr;21(4):88. doi: 10.1007/s00894-015-2621-5. Epub 2015 Mar 17. J Mol Model. 2015. PMID: 25773700
-
[A turning point in the knowledge of the structure-function-activity relations of elastin].J Soc Biol. 2001;195(2):181-93. J Soc Biol. 2001. PMID: 11727705 Review. French.
-
Helices and Sheets in vacuo.Phys Chem Chem Phys. 2007 Apr 14;9(14):1659-71. doi: 10.1039/b612615d. Epub 2007 Jan 19. Phys Chem Chem Phys. 2007. PMID: 17396176 Review.
Cited by
-
Location and Conformation of the LKα14 Peptide in Water/Ethanol Mixtures.Langmuir. 2021 Jan 12;37(1):469-477. doi: 10.1021/acs.langmuir.0c03132. Epub 2020 Dec 24. Langmuir. 2021. PMID: 33356282 Free PMC article.
-
The multifaceted helical net of amphipathic alpha-helices; the next dimension of the helical peptide wheel.Sci Prog. 2024 Oct-Dec;107(4):368504241266357. doi: 10.1177/00368504241266357. Sci Prog. 2024. PMID: 39655381 Free PMC article.
-
Liposome fusion with orthogonal coiled coil peptides as fusogens: the efficacy of roleplaying peptides.Chem Sci. 2021 Sep 22;12(41):13782-13792. doi: 10.1039/d0sc06635d. eCollection 2021 Oct 27. Chem Sci. 2021. PMID: 34760163 Free PMC article.
-
Assembly of Huntingtin headpiece into α-helical bundles.Biointerphases. 2017 May 24;12(2):02D413. doi: 10.1116/1.4984009. Biointerphases. 2017. PMID: 28539049 Free PMC article.
-
Effect of the air-water interface on the conformation of amyloid beta.Biointerphases. 2020 Dec 17;15(6):061011. doi: 10.1116/6.0000620. Biointerphases. 2020. PMID: 33334114 Free PMC article.
References
-
- Greenwald J, Riek R. Biology of Amyloid: Structure, Function, and Regulation. Structure. 2010. October;18(10):1244–1260. Available from: http://www.sciencedirect.com/science/article/pii/S0969212610003084. 10.1016/j.str.2010.08.009 - DOI - PubMed
-
- Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discovery Today. 2012. August;17(15–16):850–860. Available from: http://www.sciencedirect.com/science/article/pii/S1359644612000839. 10.1016/j.drudis.2012.03.002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

