The Aspergillus fumigatus pkcA G579R Mutant Is Defective in the Activation of the Cell Wall Integrity Pathway but Is Dispensable for Virulence in a Neutropenic Mouse Infection Model
- PMID: 26295576
- PMCID: PMC4546635
- DOI: 10.1371/journal.pone.0135195
The Aspergillus fumigatus pkcA G579R Mutant Is Defective in the Activation of the Cell Wall Integrity Pathway but Is Dispensable for Virulence in a Neutropenic Mouse Infection Model
Abstract
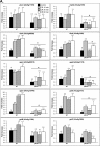
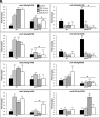
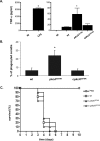
Aspergillus fumigatus is an opportunistic human pathogen, which causes the life-threatening disease, invasive pulmonary aspergillosis. In fungi, cell wall homeostasis is controlled by the conserved Cell Wall Integrity (CWI) pathway. In A. fumigatus this signaling cascade is partially characterized, but the mechanisms by which it is activated are not fully elucidated. In this study we investigated the role of protein kinase C (PkcA) in this signaling cascade. Our results suggest that pkcA is an essential gene and is activated in response to cell wall stress. Subsequently, we constructed and analyzed a non-essential A. fumigatus pkcAG579R mutant, carrying a Gly579Arg substitution in the PkcA C1B regulatory domain. The pkcAG579R mutation has a reduced activation of the downstream Mitogen-Activated Protein Kinase, MpkA, resulting in the altered expression of genes encoding cell wall-related proteins, markers of endoplasmic reticulum stress and the unfolded protein response. Furthermore, PkcAG579R is involved in the formation of proper conidial architecture and protection to oxidative damage. The pkcAG579R mutant elicits increased production of TNF-α and phagocytosis but it has no impact on virulence in a murine model of invasive pulmonary aspergillosis. These results highlight the importance of PkcA to the CWI pathway but also indicated that additional regulatory circuits may be involved in the biosynthesis and/or reinforcement of the A. fumigatus cell wall during infection.
Conflict of interest statement
Figures




Similar articles
-
Aspergillus fumigatus G-Protein Coupled Receptors GprM and GprJ Are Important for the Regulation of the Cell Wall Integrity Pathway, Secondary Metabolite Production, and Virulence.mBio. 2020 Oct 13;11(5):e02458-20. doi: 10.1128/mBio.02458-20. mBio. 2020. PMID: 33051372 Free PMC article.
-
The Aspergillus fumigatus sitA Phosphatase Homologue Is Important for Adhesion, Cell Wall Integrity, Biofilm Formation, and Virulence.Eukaryot Cell. 2015 Aug;14(8):728-44. doi: 10.1128/EC.00008-15. Epub 2015 Apr 24. Eukaryot Cell. 2015. PMID: 25911225 Free PMC article.
-
Aspergillus fumigatus MADS-Box Transcription Factor rlmA Is Required for Regulation of the Cell Wall Integrity and Virulence.G3 (Bethesda). 2016 Sep 8;6(9):2983-3002. doi: 10.1534/g3.116.031112. G3 (Bethesda). 2016. PMID: 27473315 Free PMC article.
-
Genes and molecules involved in Aspergillus fumigatus virulence.Rev Iberoam Micol. 2005 Mar;22(1):1-23. doi: 10.1016/s1130-1406(05)70001-2. Rev Iberoam Micol. 2005. PMID: 15813678 Review.
-
Molecular mechanism of Aspergillus fumigatus adherence to host constituents.Curr Opin Microbiol. 2011 Aug;14(4):375-9. doi: 10.1016/j.mib.2011.07.006. Epub 2011 Jul 23. Curr Opin Microbiol. 2011. PMID: 21784698 Free PMC article. Review.
Cited by
-
The Aspergillus flavus hacA Gene in the Unfolded Protein Response Pathway Is a Candidate Target for Host-Induced Gene Silencing.J Fungi (Basel). 2024 Oct 16;10(10):719. doi: 10.3390/jof10100719. J Fungi (Basel). 2024. PMID: 39452671 Free PMC article.
-
Aspergillus fumigatus escape mechanisms from its harsh survival environments.Appl Microbiol Biotechnol. 2024 Dec;108(1):53. doi: 10.1007/s00253-023-12952-z. Epub 2024 Jan 4. Appl Microbiol Biotechnol. 2024. PMID: 38175242 Review.
-
Survival Factor A (SvfA) Contributes to Aspergillus nidulans Pathogenicity.J Fungi (Basel). 2023 Jan 21;9(2):143. doi: 10.3390/jof9020143. J Fungi (Basel). 2023. PMID: 36836258 Free PMC article.
-
The LAMMER Kinase, LkhA, Affects Aspergillus fumigatus Pathogenicity by Modulating Reproduction and Biosynthesis of Cell Wall PAMPs.Front Cell Infect Microbiol. 2021 Oct 13;11:756206. doi: 10.3389/fcimb.2021.756206. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34722342 Free PMC article.
-
The Aspergillus fumigatus Sialidase (Kdnase) Contributes to Cell Wall Integrity and Virulence in Amphotericin B-Treated Mice.Front Microbiol. 2018 Jan 18;8:2706. doi: 10.3389/fmicb.2017.02706. eCollection 2017. Front Microbiol. 2018. PMID: 29403452 Free PMC article.
References
-
- Abad A, Fernandez-Molina JV, Bikandi J, Ramirez A, Margareto J, Sendino J, et al. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Revista iberoamericana de micologia. 2010;27(4):155–82. Epub 2010/10/27. 10.1016/j.riam.2010.10.003 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

