Investigation of Overrun-Processed Porous Hyaluronic Acid Carriers in Corneal Endothelial Tissue Engineering
- PMID: 26296087
- PMCID: PMC4546624
- DOI: 10.1371/journal.pone.0136067
Investigation of Overrun-Processed Porous Hyaluronic Acid Carriers in Corneal Endothelial Tissue Engineering
Abstract
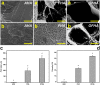
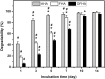
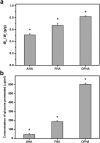
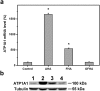
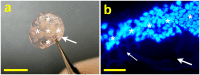
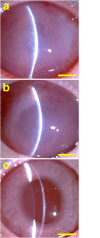
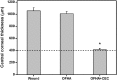
Hyaluronic acid (HA) is a linear polysaccharide naturally found in the eye and therefore is one of the most promising biomaterials for corneal endothelial regenerative medicine. This study reports, for the first time, the development of overrun-processed porous HA hydrogels for corneal endothelial cell (CEC) sheet transplantation and tissue engineering applications. The hydrogel carriers were characterized to examine their structures and functions. Evaluations of carbodiimide cross-linked air-dried and freeze-dried HA samples were conducted simultaneously for comparison. The results indicated that during the fabrication of freeze-dried HA discs, a technique of introducing gas bubbles in the aqueous biopolymer solutions can be used to enlarge pore structure and prevent dense surface skin formation. Among all the groups studied, the overrun-processed porous HA carriers show the greatest biological stability, the highest freezable water content and glucose permeability, and the minimized adverse effects on ionic pump function of rabbit CECs. After transfer and attachment of bioengineered CEC sheets to the overrun-processed HA hydrogel carriers, the therapeutic efficacy of cell/biopolymer constructs was tested using a rabbit model with corneal endothelial dysfunction. Clinical observations including slit-lamp biomicroscopy, specular microscopy, and corneal thickness measurements showed that the construct implants can regenerate corneal endothelium and restore corneal transparency at 4 weeks postoperatively. Our findings suggest that cell sheet transplantation using overrun-processed porous HA hydrogels offers a new way to reconstruct the posterior corneal surface and improve endothelial tissue function.
Conflict of interest statement
Figures


Similar articles
-
Influence of Pre-Freezing Temperature on the Corneal Endothelial Cytocompatibility and Cell Delivery Performance of Porous Hyaluronic Acid Hydrogel Carriers.Int J Mol Sci. 2015 Aug 11;16(8):18796-811. doi: 10.3390/ijms160818796. Int J Mol Sci. 2015. PMID: 26270663 Free PMC article.
-
Hyaluronic acid concentration-mediated changes in structure and function of porous carriers for corneal endothelial cell sheet delivery.Mater Sci Eng C Mater Biol Appl. 2016 Feb;59:411-419. doi: 10.1016/j.msec.2015.10.050. Epub 2015 Oct 22. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26652391
-
Carbodiimide cross-linked hyaluronic acid hydrogels as cell sheet delivery vehicles: characterization and interaction with corneal endothelial cells.J Biomater Sci Polym Ed. 2008;19(1):1-18. doi: 10.1163/156856208783227695. J Biomater Sci Polym Ed. 2008. PMID: 18177550
-
[Transplantation of corneal endothelial cells].Nippon Ganka Gakkai Zasshi. 2002 Dec;106(12):805-35; discussion 836. Nippon Ganka Gakkai Zasshi. 2002. PMID: 12610838 Review. Japanese.
-
[Cultivated corneal endothelial cell sheet transplantation in a primate model].Nippon Ganka Gakkai Zasshi. 2009 Nov;113(11):1050-9. Nippon Ganka Gakkai Zasshi. 2009. PMID: 19994583 Review. Japanese.
Cited by
-
Significance of Crosslinking Approaches in the Development of Next Generation Hydrogels for Corneal Tissue Engineering.Pharmaceutics. 2021 Feb 28;13(3):319. doi: 10.3390/pharmaceutics13030319. Pharmaceutics. 2021. PMID: 33671011 Free PMC article. Review.
-
Stem Cell-Derived Corneal Epithelium: Engineering Barrier Function for Ocular Surface Repair.Int J Mol Sci. 2025 Aug 3;26(15):7501. doi: 10.3390/ijms26157501. Int J Mol Sci. 2025. PMID: 40806627 Free PMC article. Review.
-
Applications of Hyaluronic Acid in Ophthalmology and Contact Lenses.Molecules. 2021 Apr 24;26(9):2485. doi: 10.3390/molecules26092485. Molecules. 2021. PMID: 33923222 Free PMC article. Review.
-
A hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo expansion of human corneal epithelial stem cells.Eye (Lond). 2017 Jun;31(6):962-971. doi: 10.1038/eye.2017.8. Epub 2017 Feb 17. Eye (Lond). 2017. PMID: 28211875 Free PMC article.
-
3D bioprinting as a prospective therapeutic strategy for corneal limbal epithelial stem cell deficiency.Int J Bioprint. 2023 Mar 15;9(3):710. doi: 10.18063/ijb.710. eCollection 2023. Int J Bioprint. 2023. PMID: 37273996 Free PMC article.
References
-
- Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18: 529–51. - PubMed
-
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22: 359–389. - PubMed
-
- Ide T, Nishida K, Yamato M, Sumide T, Utsumi M, Nozaki T, et al. Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature-responsive culture dishes. Biomaterials. 2006;27: 607–614. - PubMed
-
- Insler MS, Lopez JG. Microcarrier cell culture of neonatal human corneal endothelium. Curr Eye Res. 1990;9: 23–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

