The Epidemiology of Hepatitis C Virus in the Fertile Crescent: Systematic Review and Meta-Analysis
- PMID: 26296200
- PMCID: PMC4546629
- DOI: 10.1371/journal.pone.0135281
The Epidemiology of Hepatitis C Virus in the Fertile Crescent: Systematic Review and Meta-Analysis
Abstract
Objective: To characterize hepatitis C virus (HCV) epidemiology in countries of the Fertile Crescent region of the Middle East and North Africa (MENA), namely Iraq, Jordan, Lebanon, Palestine, and Syria.
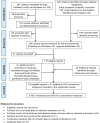
Methods: We systematically reviewed and synthesized available records of HCV incidence and prevalence following PRISMA guidelines. Meta-analyses were implemented using a DerSimonian-Laird random effects model with inverse weighting to estimate the country-specific HCV prevalence among the various at risk population groups.
Results: We identified eight HCV incidence and 240 HCV prevalence measures in the Fertile Crescent. HCV sero-conversion risk among hemodialysis patients was 9.2% in Jordan and 40.3% in Iraq, and ranged between 0% and 3.5% among other populations in Iraq over different follow-up times. Our meta-analyses estimated HCV prevalence among the general population at 0.2% in Iraq (range: 0-7.2%; 95% CI: 0.1-0.3%), 0.3% in Jordan (range: 0-2.0%; 95% CI: 0.1-0.5%), 0.2% in Lebanon (range: 0-3.4%; 95% CI: 0.1-0.3%), 0.2% in Palestine (range: 0-9.0%; 95% CI: 0.2-0.3%), and 0.4% in Syria (range: 0.3-0.9%; 95% CI: 0.4-0.5%). Among populations at high risk, HCV prevalence was estimated at 19.5% in Iraq (range: 0-67.3%; 95% CI: 14.9-24.5%), 37.0% in Jordan (range: 21-59.5%; 95% CI: 29.3-45.0%), 14.5% in Lebanon (range: 0-52.8%; 95% CI: 5.6-26.5%), and 47.4% in Syria (range: 21.0-75.0%; 95% CI: 32.5-62.5%). Genotypes 4 and 1 appear to be the dominant circulating strains.
Conclusions: HCV prevalence in the population at large appears to be below 1%, lower than that in other MENA sub-regions, and tending towards the lower end of the global range. However, there is evidence for ongoing HCV transmission within medical facilities and among people who inject drugs (PWID). Migration dynamics appear to have played a role in determining the circulating genotypes. HCV prevention efforts should be targeted, and focus on infection control in clinical settings and harm reduction among PWID.
Conflict of interest statement
Figures
References
-
- World Health Organization. Hepatitis C: fact sheet Geneva, Switzerland [updated April 2014; cited 2015 02/02]. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/.
-
- Centers for Disease Control and Prevention. Hepatitis C Atlanta, USA [updated August 1, 2013; cited 2015 02/02]. Available from: http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-disease....
-
- A SPECIAL MEETING REVIEW EDITION: Advances in the Treatment of Hepatitis C Virus Infection from The Liver Meeting 2013: The 64th Annual Meeting of the American Association for the Study of Liver DiseasesNovember 1–5, 2013 • Washington DCSpecial Reporting on:• Simeprevir plus Sofosbuvir with or without Ribavirin Produces High SVR Rates in Genotype 1 HCV Infection• Novel Interferon- and Ribavirin-Free Regimen Results in SVR12 Rates of Over 90% in HCV Genotype 1b Infection• Studies Confirm Efficacy of Adjunctive Simeprevir in Difficult-to-Treat HCV Genotype 1 Subpopulations• All-Oral Therapy with Sofosbuvir Plus Ribavirin Produces High SVR Rates in Patients Coinfected with HCV and HIV• Faldaprevir Combined with Pegylated Interferon and Ribavirin Demonstrates High Efficacy in DifficuIt-to-Treat HCV Infection• Once Daily Sofosbuvir/Ledipasvir Combination Elicits Rapid Decline in HCV RNA Gastroenterology & Hepatology. 2014;10(1 Suppl 1):1–19. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials