Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial
- PMID: 26296511
- PMCID: PMC4567458
- DOI: 10.1212/WNL.0000000000001930
Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial
Abstract
Objective: To assess efficacy and safety of adjunctive perampanel in patients with drug-resistant, primary generalized tonic-clonic (PGTC) seizures in idiopathic generalized epilepsy (IGE).
Methods: In this multicenter, double-blind study (ClinicalTrials.gov identifier: NCT01393743; funded by Eisai Inc.), patients 12 years or older with PGTC seizures and IGE were randomized to placebo or perampanel during a 4-week titration period (perampanel up titrated from 2 to 8 mg/d, or highest tolerated dose) and 13-week maintenance period. The primary endpoint was percent change in PGTC seizure frequency per 28 days (titration plus maintenance vs baseline). The key secondary endpoint (primary endpoint for European Union registration) was 50% PGTC seizure responder rate (patients achieving $50% reduction in PGTC seizure frequency; maintenance vs baseline). Treatment-emergent adverse events were monitored.
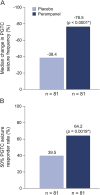
Results: Of 164 randomized patients, 162 comprised the full analysis set (placebo, 81; perampanel, 81). Compared with placebo, perampanel conferred a greater median percent change in PGTC seizure frequency per 28 days (238.4%vs 276.5%; p , 0.0001) and greater 50%PGTC seizure responder rate (39.5% vs 64.2%; p 5 0.0019). During maintenance, 12.3% of placebo treated patients and 30.9%of perampanel-treated patients achieved PGTC seizure freedom. For the safety analysis (placebo, 82; perampanel, 81), the most frequent treatment-emergent adverse events with perampanel were dizziness (32.1%) and fatigue (14.8%).
Conclusions: Adjunctive perampanel was well tolerated and improved control of drug-resistant PGTC seizures in patients with IGE.
Classification of evidence: This study provides Class I evidence that adjunctive perampanel reduces PGTC seizure frequency, compared with placebo, in patients with drug-resistant PGTC seizures in IGE.
Figures
References
-
- Rheims S, Ryvlin P. Pharmacotherapy for tonic-clonic seizures. Expert Opin Pharmacother 2014;15:1417–1426. - PubMed
-
- Hanada T, Yang H, Ido K, Laurenza A. AMPA Receptor Antagonists for the Treatment of CNS Disorders: Antiepileptics and Beyond. Frontiers in Clinical Drug Research (Volume 3): CNS and Neurological Disorders. Sharjah, UAE: Bentham Science Publishers; 2015:34–76.
-
- Durmuller N, Craggs M, Meldrum BS. The effect of the non-NMDA receptor antagonist GYKI 52466 and NBQX and the competitive NMDA receptor antagonist D-CPPene on the development of amygdala kindling and on amygdala-kindled seizures. Epilepsy Res 1994;17:167–174. - PubMed
-
- Kodama M, Yamada N, Sato K, et al. Effects of YM90K, a selective AMPA receptor antagonist, on amygdala-kindling and long-term hippocampal potentiation in the rat. Eur J Pharmacol 1999;374:11–19. - PubMed
-
- Wu T, Nagaya Y, Hanada T. Pharmacodynamic and pharmacokinetic interactions of perampanel and other antiepileptic drugs in a rat amygdala kindling model. Seizure 2014;23:732–739. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
