Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation Approaches: A Systematic Review of Published Models, Applications, and Model Verification
- PMID: 26296709
- PMCID: PMC4613950
- DOI: 10.1124/dmd.115.065920
Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation Approaches: A Systematic Review of Published Models, Applications, and Model Verification
Abstract
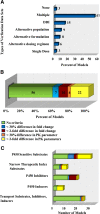
Modeling and simulation of drug disposition has emerged as an important tool in drug development, clinical study design and regulatory review, and the number of physiologically based pharmacokinetic (PBPK) modeling related publications and regulatory submissions have risen dramatically in recent years. However, the extent of use of PBPK modeling by researchers, and the public availability of models has not been systematically evaluated. This review evaluates PBPK-related publications to 1) identify the common applications of PBPK modeling; 2) determine ways in which models are developed; 3) establish how model quality is assessed; and 4) provide a list of publically available PBPK models for sensitive P450 and transporter substrates as well as selective inhibitors and inducers. PubMed searches were conducted using the terms "PBPK" and "physiologically based pharmacokinetic model" to collect published models. Only papers on PBPK modeling of pharmaceutical agents in humans published in English between 2008 and May 2015 were reviewed. A total of 366 PBPK-related articles met the search criteria, with the number of articles published per year rising steadily. Published models were most commonly used for drug-drug interaction predictions (28%), followed by interindividual variability and general clinical pharmacokinetic predictions (23%), formulation or absorption modeling (12%), and predicting age-related changes in pharmacokinetics and disposition (10%). In total, 106 models of sensitive substrates, inhibitors, and inducers were identified. An in-depth analysis of the model development and verification revealed a lack of consistency in model development and quality assessment practices, demonstrating a need for development of best-practice guidelines.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Abduljalil K, Cain T, Humphries H, Rostami-Hodjegan A. (2014) Deciding on success criteria for predictability of pharmacokinetic parameters from in vitro studies: an analysis based on in vivo observations. Drug Metab Dispos 42:1478–1484. - PubMed
-
- Agoram B, Woltosz WS, Bolger MB. (2001) Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev 50 (Suppl 1):S41–S67. - PubMed
-
- Almond LM, Yang J, Jamei M, Tucker GT, Rostami-Hodjegan A. (2009) Towards a quantitative framework for the prediction of DDIs arising from cytochrome P450 induction. Curr Drug Metab 10:420–432. - PubMed
-
- Andrew MA, Hebert MF, Vicini P. (2008). Physiologically based pharmacokinetic model of midazolam disposition during pregnancy. Conf Proc IEEE Eng Med Biol Soc 2008:5454–5457. - PubMed
-
- Ball K, Bouzom F, Scherrmann JM, Walther B, Declèves X. (2014) Comparing translational population-PBPK modelling of brain microdialysis with bottom-up prediction of brain-to-plasma distribution in rat and human. Biopharm Drug Dispos 35:485–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

