Comparison of adipose tissue- and bone marrow- derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats
- PMID: 26296856
- PMCID: PMC4546321
- DOI: 10.1186/s13287-015-0142-x
Comparison of adipose tissue- and bone marrow- derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats
Abstract
Introduction: Doxorubicin (DOX) is a well-known anticancer drug. However its clinical use has been limited due to cardiotoxic effects. One of the major concerns with DOX therapy is its toxicity in patients who are frail, particularly diabetics. Several studies suggest that mesenchymal stem cells (MSCs) have the potential to restore cardiac function after DOX-induced injury. However, limited data are available on the effects of MSC therapy on DOX-induced cardiac dysfunction in diabetics. Our objective was to test the efficacy of bone marrow-derived (BM-MSCs) and adipose-derived MSCs (AT-MSCs) from age-matched humans in a non-immune compromised rat model.
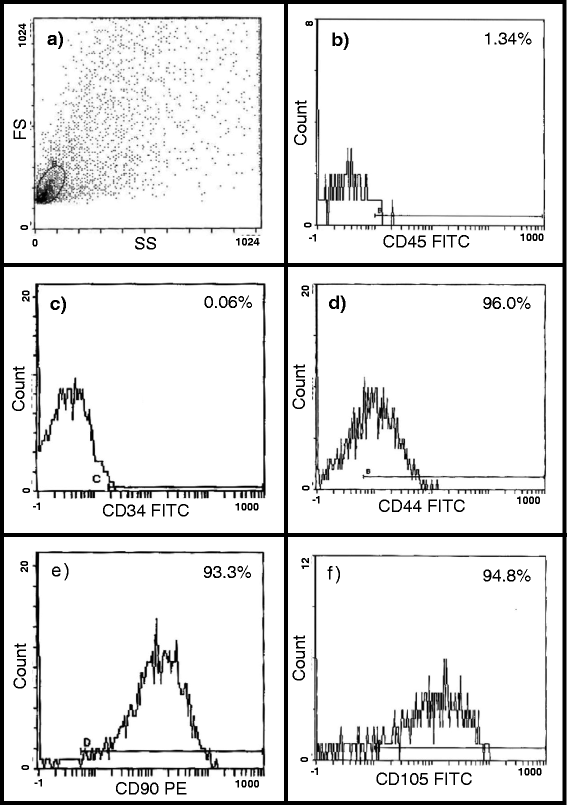
Methods: Diabetes mellitus was induced in rats by streptozotocin injection (STZ, 65 mg/kg b.w, i.p.). Diabetic rats were treated with DOX (doxorubicin hydrochloride, 2.5 mg/kg b.w, i.p) 3 times/wk for 2 weeks (DOX group); or with DOX+ GFP labelled BM-MSCs (2x106cells, i.v.) or with DOX + GFP labelled AT-MSCs (2x106cells, i.v.). Echocardiography and Langendorff perfusion analyses were carried out to determine the heart function. Immunostaining and western blot analysis of the heart tissue was carried out for CD31 and to assess inflammation and fibrosis. Statistical analysis was carried out using SPSS and data are expressed as mean ± SD.
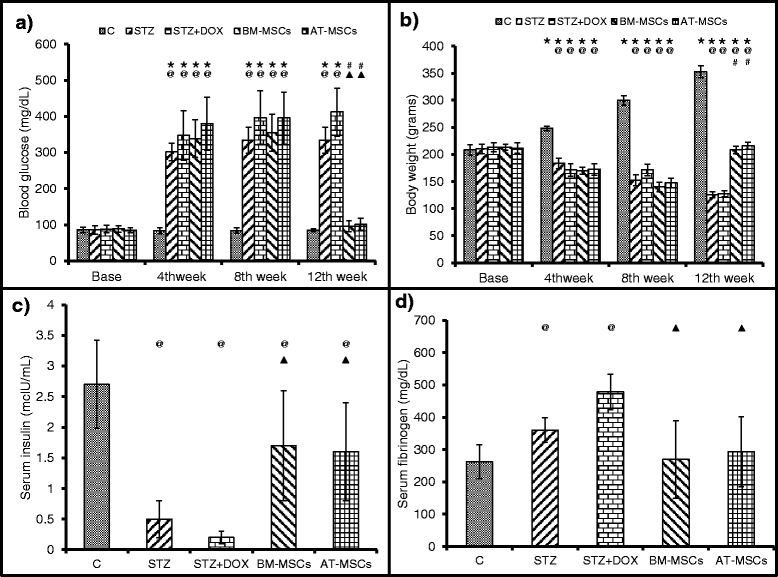
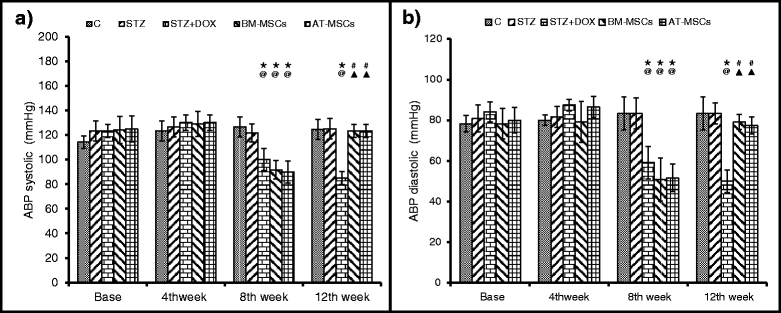
Results: Glucose levels in the STZ treated groups were significantly greater than control group. After 4 weeks of intravenous injection, the presence of injected MSCs in the heart was confirmed through fluorescent microscopy and real time PCR for ALU transcripts. Both BM-MSCs and AT-MSCs injection prevented DOX-induced deterioration of %FS, LVDP, dp/dt max and rate pressure product. Staining for CD31 showed a significant increase in the number of capillaries in BM-MSCs and AT-MSCs treated animals in comparison to DOX treated group. Assessment of the inflammation and fibrosis revealed a marked reduction in the DOX-induced increase in immune cell infiltration, collagen deposition and αSMA in the BM-MSCs and AT-MSCs groups.
Conclusions: In conclusion BM-MSCs and AT-MSCs were equally effective in mitigating DOX-induced cardiac damage by promoting angiogenesis, decreasing the infiltration of immune cells and collagen deposition.
Figures




References
-
- International Diabetes Federation. IDF Diabetes Atlas. In: Guariguata L, Nolan T, Beagley J, Linnenkamp U, Jacqmain O, editors. International Diabetes Federation. 6th ed. 2013. Chapter 2: 32-49.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

