Short-Term, High-Dose Fish Oil Supplementation Increases the Production of Omega-3 Fatty Acid-Derived Mediators in Patients With Peripheral Artery Disease (the OMEGA-PAD I Trial)
- PMID: 26296857
- PMCID: PMC4599461
- DOI: 10.1161/JAHA.115.002034
Short-Term, High-Dose Fish Oil Supplementation Increases the Production of Omega-3 Fatty Acid-Derived Mediators in Patients With Peripheral Artery Disease (the OMEGA-PAD I Trial)
Abstract
Background: Patients with peripheral artery disease (PAD) experience significant morbidity and mortality. The OMEGA-PAD I Trial, a randomized, double-blinded, placebo-controlled trial, addressed the hypothesis that short-duration, high-dose n-3 polyunsaturated fatty acids (n-3 PUFA) oral supplementation improves endothelial function and inflammation in PAD.
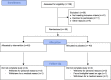
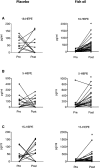
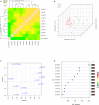
Methods and results: Eighty patients with stable claudication received 4.4 g of fish oil or placebo for 1 month. The primary end point was endothelial function as measured by brachial artery flow-mediated vasodilation. Secondary end points included biomarkers of inflammation, n-3 polyunsaturated fatty acids metabolome changes, lipid profile, and walking impairment questionnaires. Although there was a significant increase in FMD in the fish oil group following treatment (0.7±1.8% increase from baseline, P=0.04), this response was not different then the placebo group (0.6±2.5% increase from baseline, P=0.18; between-group P=0.86) leading to a negative finding for the primary endpoint. There was, however, a significant reduction in triglycerides (fish oil: -34±46 mg/dL, P<0.001; placebo -10±43 mg/dL, P=0.20; between-group differential P-value: 0.02), and an increase in the omega-3 index of 4±1% (P<0.001) in the fish oil group (placebo 0.1±0.9%, P=0.49; between-group P<0.0001). We observed a significant increase in the production of pathway markers of specialized pro-resolving mediators generated from n-3 polyunsaturated fatty acids in the fish oil group.
Conclusions: High-dose, short-duration fish oil supplementation did not lead to a different response in the primary end point of endothelial function between the treatment and placebo group, but improved serum triglycerides and increased the production of downstream n-3 polyunsaturated fatty acids-derived products and mediators in patients with PAD.
Clinical trial registration: URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01310270.
Keywords: fish oil; n‐3 polyunsaturated fatty acids; peripheral artery disease; specialized pro‐resolving mediators; vascular function.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures

References
-
- Cotter G, Cannon CP, McCabe CH, Michowitz Y, Kaluski E, Charlesworth A, Milo O, Bentley J, Blatt A, Krakover R, Zimlichman R, Reisin L, Marmor A, Lewis B, Vered Z, Caspi A, Braunwald E. Prior peripheral arterial disease and cerebrovascular disease are independent predictors of adverse outcome in patients with acute coronary syndromes: are we doing enough? Results from the Orbofiban in Patients with Unstable Coronary Syndromes-Thrombolysis in Myocardial Infarction (OPUS-TIMI) 16 study. Am Heart J. 2003;145:622–627. - PubMed
-
- Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. - PubMed
-
- Vidula H, Tian L, Liu K, Criqui MH, Ferrucci L, Pearce WH, Greenland P, Green D, Tan J, Garside DB, Guralnik J, Ridker PM, Rifai N, McDermott MM. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med. 2008;148:85–93. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

