Safety and tolerability of guadecitabine (SGI-110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: a multicentre, randomised, dose-escalation phase 1 study
- PMID: 26296954
- PMCID: PMC5557041
- DOI: 10.1016/S1470-2045(15)00038-8
Safety and tolerability of guadecitabine (SGI-110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: a multicentre, randomised, dose-escalation phase 1 study
Abstract
Background: Hypomethylating agents are used to treat cancers driven by aberrant DNA methylation, but their short half-life might limit their activity, particularly in patients with less proliferative diseases. Guadecitabine (SGI-110) is a novel hypomethylating dinucleotide of decitabine and deoxyguanosine resistant to degradation by cytidine deaminase. We aimed to assess the safety and clinical activity of subcutaneously given guadecitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome.
Methods: In this multicentre, open-label, phase 1 study, patients from nine North American medical centres with myelodysplastic syndrome or acute myeloid leukaemia that was refractory to or had relapsed after standard treatment were randomly assigned (1:1) to receive subcutaneous guadecitabine, either once-daily for 5 consecutive days (daily × 5), or once-weekly for 3 weeks, in a 28-day treatment cycle. Patients were stratified by disease. A 3 + 3 dose-escalation design was used in which we treated patients with guadecitabine doses of 3-125 mg/m(2) in separate dose-escalation cohorts. A twice-weekly treatment schedule was added to the study after a protocol amendment. The primary objective was to assess safety and tolerability of guadecitabine, determine the maximum tolerated and biologically effective dose, and identify the recommended phase 2 dose of guadecitabine. Safety analyses included all patients who received at least one dose of guadecitabine. Pharmacokinetic and pharmacodynamic analyses to determine the biologically effective dose included all patients for whom samples were available. This study is registered with ClinicalTrials.gov, number NCT01261312.
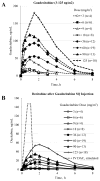
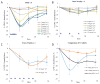
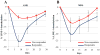
Findings: Between Jan 4, 2011, and April 11, 2014, we enrolled and treated 93 patients: 35 patients with acute myeloid leukaemia and nine patients with myelodysplastic syndrome in the daily × 5 dose-escalation cohorts, 28 patients with acute myeloid leukaemia and six patients with myelodysplastic syndrome in the once-weekly dose-escalation cohorts, and 11 patients with acute myeloid leukaemia and four patients with myelodysplastic syndrome in the twice-weekly dose-escalation cohorts. The most common grade 3 or higher adverse events were febrile neutropenia (38 [41%] of 93 patients), pneumonia (27 [29%] of 93 patients), thrombocytopenia (23 [25%] of 93 patients), anaemia (23 [25%] of 93 patients), and sepsis (16 [17%] of 93 patients). The most common serious adverse events were febrile neutropenia (29 [31%] of 93 patients), pneumonia (26 [28%] of 93 patients), and sepsis (16 [17%] of 93 patients). Six of the 74 patients with acute myeloid leukaemia and six of the 19 patients with myelodysplastic syndrome had a clinical response to treatment. Two dose-limiting toxicities were noted in patients with myelodysplastic syndrome at 125 mg/m(2) daily × 5, thus the maximum tolerated dose in patients with myelodysplastic syndrome was 90 mg/m(2) daily × 5. The maximum tolerated dose was not reached in patients with acute myeloid leukaemia. Potent dose-related DNA demethylation occurred on the daily × 5 regimen, reaching a plateau at 60 mg/m(2) (designated as the biologically effective dose).
Interpretation: Guadecitabine given subcutaneously at 60 mg/m(2) daily × 5 is well tolerated and is clinically and biologically active in patients with myelodysplastic syndrome and acute myeloid leukaemia. Guadecitabine 60 mg/m(2) daily × 5 is the recommended phase 2 dose, and these findings warrant further phase 2 studies.
Funding: Astex Pharmaceuticals, Stand Up To Cancer.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
J-PI has served as a consultant for GSK, TEVA, Astex and Janssen, has served as an advisory board member for BI and has received research funding from Astex.
GR has received personal fees from AstraZeneca, Celgene, Boehringer Ingelheim, Roche, Novartis, Astex, Agios, Shire, and GlaxoSmithKline, and has received research funding from Astex.
DR: None
EJ: None
WS: None
CO has served on a speaker’s bureau for Celgene and as a scientific advisory board member for Incyte and Alexion.
KY has served as a consultant for Methylgene; received honoraria from Celgene Canada; and received research grants from Astex Pharmaceuticals, Celator Pharmaceuticals, Roche, Karyopharm, Oncoethix, Genentech, Boehringer Ingelheim, Novartis Pharmaceuticals, and GlaxoSmithKline.
RT: None
EG has served as a consultant for Ariad, has received honoraria from Celgene and Alexion, and has received research funding from Astex.
KW: None
ND: None
WC has received research funding from Astex.
SN, PT, AO, YH, JL, and MA are employees of Astex Pharmaceuticals, Inc.
HK has received research grants from Astex Pharmaceuticals, Inc.
Figures

 .
.
Comment in
-
Guadecitabine for AML and MDS: hype or hope?Lancet Oncol. 2015 Sep;16(9):1009-1011. doi: 10.1016/S1470-2045(15)00095-9. Epub 2015 Aug 19. Lancet Oncol. 2015. PMID: 26296955 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

