Differential Growth in Periclinal and Anticlinal Walls during Lobe Formation in Arabidopsis Cotyledon Pavement Cells
- PMID: 26296967
- PMCID: PMC4815096
- DOI: 10.1105/tpc.114.126664
Differential Growth in Periclinal and Anticlinal Walls during Lobe Formation in Arabidopsis Cotyledon Pavement Cells
Abstract
Lobe development in the epidermal pavement cells of Arabidopsis thaliana cotyledons and leaves is thought to take place via tip-like growth on the concave side of lobes driven by localized concentrations of actin filaments and associated proteins, with a predicted role for cortical microtubules in establishing the direction of restricted growth at the convex side. We used homologous landmarks fixed to the outer walls of pavement cells and thin-plate spline analysis to demonstrate that lobes form by differential growth of both the anticlinal and periclinal walls. Most lobes formed within the first 24 h of the cotyledons unfurling, during the period of rapid cell expansion. Cortical microtubules adjacent to the periclinal wall were persistently enriched at the convex side of lobes during development where growth was anisotropic and were less concentrated or absent at the concave side where growth was promoted. Alternating microtubule-enriched and microtubule-free zones at the periclinal wall in neighboring cells predicted sites of new lobes. There was no particular arrangement of cortical actin filaments that could predict where lobes would form. However, drug studies demonstrate that both filamentous actin and microtubules are required for lobe formation.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures
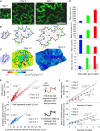

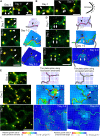
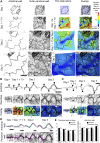


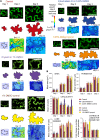
Comment in
-
Tracking Pavement Cells through Space and Time: Microtubules Define Positions of Lobe Formation.Plant Cell. 2015 Sep;27(9):2317. doi: 10.1105/tpc.15.00709. Epub 2015 Aug 21. Plant Cell. 2015. PMID: 26296965 Free PMC article. No abstract available.
References
-
- Arganda-Carreras I., Fernández-González R., Muñoz-Barrutia A., Ortiz-De-Solorzano C. (2010). 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc. Res. Tech. 73: 1019–1029. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

