Signaling Over Distances
- PMID: 26297514
- PMCID: PMC4739662
- DOI: 10.1074/mcp.R115.052753
Signaling Over Distances
Abstract
Neurons are extremely polarized cells. Axon lengths often exceed the dimension of the neuronal cell body by several orders of magnitude. These extreme axonal lengths imply that neurons have mastered efficient mechanisms for long distance signaling between soma and synaptic terminal. These elaborate mechanisms are required for neuronal development and maintenance of the nervous system. Neurons can fine-tune long distance signaling through calcium wave propagation and bidirectional transport of proteins, vesicles, and mRNAs along microtubules. The signal transmission over extreme lengths also ensures that information about axon injury is communicated to the soma and allows for repair mechanisms to be engaged. This review focuses on the different mechanisms employed by neurons to signal over long axonal distances and how signals are interpreted in the soma, with an emphasis on proteomic studies. We also discuss how proteomic approaches could help further deciphering the signaling mechanisms operating over long distance in axons.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

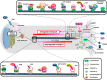
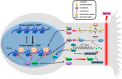
References
-
- Panayotis N., Karpova A., Kreutz M. R., and Fainzilber M. (2015) Macromolecular transport in synapse to nucleus communication. Trends Neurosci. 38, 108–116 - PubMed
-
- Rishal I., and Fainzilber M. (2014) Axon-soma communication in neuronal injury. Nat. Rev. Neurosci. 15, 32–42 - PubMed
-
- Bradke F., Fawcett J. W., and Spira M. E. (2012) Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat. Rev. Neurosci. 13, 183–193 - PubMed
-
- Detrait E., Eddleman C. S., Yoo S., Fukuda M., Nguyen M. P., Bittner G. D., and Fishman H. M. (2000) Axolemmal repair requires proteins that mediate synaptic vesicle fusion. J. Neurobiol. 44, 382–391 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

