Complex I dysfunction underlies the glycolytic switch in pulmonary hypertensive smooth muscle cells
- PMID: 26298201
- PMCID: PMC4556771
- DOI: 10.1016/j.redox.2015.07.016
Complex I dysfunction underlies the glycolytic switch in pulmonary hypertensive smooth muscle cells
Abstract
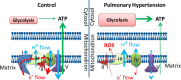
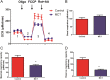
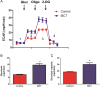
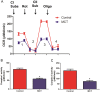
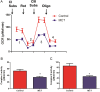
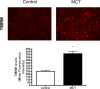
ATP is essential for cellular function and is usually produced through oxidative phosphorylation. However, mitochondrial dysfunction is now being recognized as an important contributing factor in the development cardiovascular diseases, such as pulmonary hypertension (PH). In PH there is a metabolic change from oxidative phosphorylation to mainly glycolysis for energy production. However, the mechanisms underlying this glycolytic switch are only poorly understood. In particular the role of the respiratory Complexes in the mitochondrial dysfunction associated with PH is unresolved and was the focus of our investigations. We report that smooth muscle cells isolated from the pulmonary vessels of rats with PH (PH-PASMC), induced by a single injection of monocrotaline, have attenuated mitochondrial function and enhanced glycolysis. Further, utilizing a novel live cell assay, we were able to demonstrate that the mitochondrial dysfunction in PH-PASMC correlates with deficiencies in the activities of Complexes I-III. Further, we observed that there was an increase in mitochondrial reactive oxygen species generation and mitochondrial membrane potential in the PASMC isolated from rats with PH. We further found that the defect in Complex I activity was due to a loss of Complex I assembly, although the assembly of Complexes II and III were both maintained. Thus, we conclude that loss of Complex I assembly may be involved in the switch of energy metabolism in smooth muscle cells to glycolysis and that maintaining Complex I activity may be a potential therapeutic target for the treatment of PH.
Keywords: Electron transport chain; Mitochondria; Pulmonary hypertension; Warburg effect.
Copyright © 2015. Published by Elsevier B.V.
Figures


References
-
- Owens G.K., Kumar M.S., Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. - PubMed
-
- Hao H., Gabbiani G., Bochaton-Piallat M.L. Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler. Thromb. Vasc. Biol. 2003;23:1510–1520. - PubMed
-
- Lincoln T.M., Dey N.B., Boerth N.J., Cornwell T.L., Soff G.A. Nitric oxide-cyclic GMP pathway regulates vascular smooth muscle cell phenotypic modulation: implications in vascular diseases. Acta Physiol. Scand. 1998;164:507–515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 HL010313/HL/NHLBI NIH HHS/United States
- F32HL10313/HL/NHLBI NIH HHS/United States
- P01HL0101902/HL/NHLBI NIH HHS/United States
- P01 HL101902/HL/NHLBI NIH HHS/United States
- 14SDG20480354/AHA/American Heart Association-American Stroke Association/United States
- R01 HL061284/HL/NHLBI NIH HHS/United States
- HL67841/HL/NHLBI NIH HHS/United States
- HL60190/HL/NHLBI NIH HHS/United States
- HL61284/HL/NHLBI NIH HHS/United States
- R01 HL132918/HL/NHLBI NIH HHS/United States
- R01 HL067841/HL/NHLBI NIH HHS/United States
- R01 HL060190/HL/NHLBI NIH HHS/United States
- T32 HD049303/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

