Thymidine Phosphorylase in Cancer; Enemy or Friend?
- PMID: 26298314
- PMCID: PMC4842186
- DOI: 10.1007/s12307-015-0173-y
Thymidine Phosphorylase in Cancer; Enemy or Friend?
Abstract
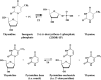
Thymidine phosphorylase (TP) is a nucleoside metabolism enzyme that plays an important role in the pyrimidine pathway.TP catalyzes the conversion of thymidine to thymine and 2-deoxy-α-D-ribose-1-phosphate (dRib-1-P). Although this reaction is reversible, the main metabolic function of TP is catabolic. TP is identical to the angiogenic factor platelet-derived endothelial-cell growth factor (PD-ECGF). TP is overexpressed in several human cancers in response to cellular stressful conditions like hypoxia, acidosis, chemotherapy and radiotherapy. TP has been shown to promote tumor angiogenesis, invasion, metastasis, evasion of the immune-response and resistance to apoptosis. Some of the biological effects of TP are dependent on its enzymatic activity, while others are mediated through cytokines like interleukin 10 (IL-10), basic fibroblast growth factor (bFGF) and tumour necrosis factor α (TNFα). Interestingly, TP also plays a role in cancer treatment through its role in the conversion of the oral fluoropyrimidine capecitabine into its active form 5-FU. TP is a predictive marker for fluoropyrimidine response. Given its various biological functions in cancer progression, TP is a promising target in cancer treatment. Further translational research is required in this area.
Keywords: Angiogenesis; Hallmarks of cancer; Predictive biomarkers; Thymidine phosphorylase.
Figures

Similar articles
-
2-Deoxy-D-ribose, a downstream mediator of thymidine phosphorylase, regulates tumor angiogenesis and progression.Anticancer Agents Med Chem. 2009 Feb;9(2):239-45. doi: 10.2174/187152009787313846. Anticancer Agents Med Chem. 2009. PMID: 19199868 Review.
-
Suppression of thymidine phosphorylase-mediated angiogenesis and tumor growth by 2-deoxy-L-ribose.Cancer Res. 2002 May 15;62(10):2834-9. Cancer Res. 2002. PMID: 12019161
-
The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression.Cancer Sci. 2004 Nov;95(11):851-7. doi: 10.1111/j.1349-7006.2004.tb02193.x. Cancer Sci. 2004. PMID: 15546501 Free PMC article. Review.
-
Inhibition of metastasis of tumor cells overexpressing thymidine phosphorylase by 2-deoxy-L-ribose.Cancer Res. 2004 Mar 1;64(5):1794-801. doi: 10.1158/0008-5472.can-03-2597. Cancer Res. 2004. PMID: 14996742
-
Prevention of hypoxia-induced apoptosis by the angiogenic factor thymidine phosphorylase.Biochem Biophys Res Commun. 1998 Dec 30;253(3):797-803. doi: 10.1006/bbrc.1998.9852. Biochem Biophys Res Commun. 1998. PMID: 9918807
Cited by
-
Outcomes of Adjuvant Oral versus Intravenous Fluoropyrimidines for High-Risk Stage II or Stage III Colon Adenocarcinoma: A Propensity Score-Matched, Nationwide, Population-Based Cohort Study.J Cancer. 2020 Apr 12;11(14):4157-4165. doi: 10.7150/jca.42404. eCollection 2020. J Cancer. 2020. PMID: 32368298 Free PMC article.
-
1,3,4-Oxadiazole: An Emerging Scaffold to Inhibit the Thymidine Phosphorylase as an Anticancer Agent.Curr Med Chem. 2024;31(38):6227-6250. doi: 10.2174/0929867331666230712113943. Curr Med Chem. 2024. PMID: 37438902 Review.
-
Demethoxycurcumin-Loaded Chitosan Nanoparticle Downregulates DNA Repair Pathway to Improve Cisplatin-Induced Apoptosis in Non-Small Cell Lung Cancer.Molecules. 2018 Dec 5;23(12):3217. doi: 10.3390/molecules23123217. Molecules. 2018. PMID: 30563166 Free PMC article.
-
Tumor-associated macrophages (TAMs) as biomarkers for gastric cancer: A review.Chronic Dis Transl Med. 2018 Sep 3;4(3):156-163. doi: 10.1016/j.cdtm.2018.07.001. eCollection 2018 Sep. Chronic Dis Transl Med. 2018. PMID: 30276362 Free PMC article.
-
Thymidine phosphorylase affects clinical outcome following surgery and mRNA expression levels of four key enzymes for 5-fluorouracil metabolism in patients with stage I and II non-small cell lung cancer.Mol Clin Oncol. 2018 Dec;9(6):640-646. doi: 10.3892/mco.2018.1726. Epub 2018 Sep 25. Mol Clin Oncol. 2018. PMID: 30546894 Free PMC article.
References
-
- Friedkin M, Roberts D. The enzymatic synthesis of nucleosides. I. thymidine phosphorylase in mammalian tissue. J Biol Chem. 1954;207(1):254–256. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

