Venlafaxine for neuropathic pain in adults
- PMID: 26298465
- PMCID: PMC6481532
- DOI: 10.1002/14651858.CD011091.pub2
Venlafaxine for neuropathic pain in adults
Abstract
Background: Neuropathic pain, which is caused by nerve damage, is increasing in prevalence worldwide. This may reflect improved diagnosis, or it may be due to increased incidence of diabetes-associated neuropathy, linked to increasing levels of obesity. Other types of neuropathic pain include post-herpetic neuralgia, trigeminal neuralgia, and neuralgia caused by chemotherapy. Antidepressant drugs are sometimes used to treat neuropathic pain; however, their analgesic efficacy is unclear. A previous Cochrane review that included all antidepressants for neuropathic pain is being replaced by new reviews of individual drugs examining chronic neuropathic pain in the first instance. Venlafaxine is a reasonably well-tolerated antidepressant and is a serotonin reuptake inhibitor and weak noradrenaline reuptake inhibitor. Although not licensed for the treatment of chronic or neuropathic pain in most countries, it is sometimes used for this indication.
Objectives: To assess the analgesic efficacy of, and the adverse effects associated with the clinical use of, venlafaxine for chronic neuropathic pain in adults.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via The Cochrane Library, and MEDLINE and EMBASE via Ovid up to 14 August 2014. We reviewed the bibliographies of any randomised trials identified and review articles, contacted authors of one excluded study and searched www.clinicaltrials.gov to identify additional published or unpublished data. We also searched the meta-Register of controlled trials (mRCT) (www.controlled-trials.com/mrct) and the WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) for ongoing trials but did not find any relevant trials.
Selection criteria: We included randomised, double-blind studies of at least two weeks' duration comparing venlafaxine with either placebo or another active treatment in chronic neuropathic pain in adults. All participants were aged 18 years or over and all included studies had at least 10 participants per treatment arm. We only included studies with full journal publication.
Data collection and analysis: Three review authors independently extracted data using a standard form and assessed study quality. We intend to analyse data in three tiers of evidence as described by Hearn 2014, but did not find any first-tier evidence (ie evidence meeting current best standards, with minimal risk of bias) or second-tier evidence, that was considered at some risk of bias but with adequate participant numbers (at least 200 in the comparison). Third-tier evidence is that arising from studies with small numbers of participants; studies of short duration, studies that are likely to be of limited clinical utility due to other limitations, including selection bias and attrition bias; or a combination of these.
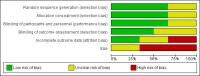
Main results: We found six randomised, double-blind trials of at least two weeks' duration eligible for inclusion. These trials included 460 participants with neuropathic pain, with most participants having painful diabetic neuropathy. Four studies were of cross-over design and two were parallel trials. Only one trial was both parallel design and placebo-controlled. Mean age of participants ranged from 48 to 59 years. In three studies (Forssell 2004, Jia 2006 and Tasmuth 2002), only mean data were reported. Comparators included placebo, imipramine, and carbamazepine and duration of treatment ranged from two to eight weeks. The risk of bias was considerable overall in the review, especially due to the small size of most studies and due to attrition bias. Four of the six studies reported some positive benefit for venlafaxine. In the largest study by Rowbotham, 2004, 56% of participants receiving venlafaxine 150 to 225 mg achieved at least a 50% reduction in pain intensity versus 34% of participants in the placebo group and the number needed to treat for an additional beneficial outcome was 4.5. However, this study was subject to significant selection bias. Known adverse effects of venlafaxine, including somnolence, dizziness, and mild gastrointestinal problems, were reported in all studies but were not particularly problematic and, overall, adverse effects were equally prominent in placebo or other active comparator groups.
Authors' conclusions: We found little compelling evidence to support the use of venlafaxine in neuropathic pain. While there was some third-tier evidence of benefit, this arose from studies that had methodological limitations and considerable risk of bias. Placebo effects were notably strong in several studies. Given that effective drug treatments for neuropathic pain are in current use, there is no evidence to revise prescribing guidelines to promote the use of venlafaxine in neuropathic pain. Although venlafaxine was generally reasonably well tolerated, there was some evidence that it can precipitate fatigue, somnolence, nausea, and dizziness in a minority of people.
Conflict of interest statement
HCG has no relevant conflicts of interest. HCG was a recipient of a Health Research Board Cochrane Fellowship which allowed her protected time to complete this review. She has other aligned pharmacological research interests that are funded by L'Air Liquide and the Mater Private Hospital, but this funding does not constitute a competing interest and did not influence the outcome of this review in any manner.
RMG has no relevant conflicts of interest.
DJB has no relevant conflicts of interest.
MB has no relevant conflicts of interest.
MCH has no relevant conflicts of interest.
Figures
Update of
References
References to studies included in this review
Forssell 2004 {published data only}
-
- Forssell H, Tasmuth T, Tenovuo O, Hampf G, Kalso E. Venlafaxine in the treatment of atypical facial pain: a randomized controlled trial. Journal of Orofacial Pain 2004;18(2):131‐7. [PUBMED: 15250433] - PubMed
Jia 2006 {published data only}
-
- Jia H‐Y, Li Q‐F, Song D‐P, An Z‐M, Liu Y‐P, Ran X‐W, et al. Effects of venlafaxine and carbamazepine for painful peripheral diabetic neuropathy: a randomized, double‐blind and double‐dummy, controlled multi‐center trial. Chinese Journal of Evidence‐Based Medicine 2006;6(5):321‐7.
Rowbotham 2004 {published data only}
-
- Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double‐blind, placebo‐controlled study. Pain 2004;110(3):697‐706. - PubMed
Sindrup 2003 {published data only}
-
- Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology 2003;60(8):1284‐9. - PubMed
Tasmuth 2002 {published data only}
-
- Tasmuth T, Härtel B, Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. European Journal of Pain 2002;6(1):17‐24. - PubMed
Yucel 2005 {published data only}
-
- Yucel A, Ozyalcin S, Talu GK, Kiziltan E, Yucel B, Andersen OK, et al. The effect of venlafaxine on ongoing and experimentally induced pain in neuropathic pain patients: a double blind, placebo controlled study. European Journal of Pain 2005;9(4):407‐16. - PubMed
References to studies excluded from this review
Amr 2010 {published data only}
-
- Amr YM, Yousef AA. Evaluation of the efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clinical Journal of Pain 2010;26(5):381‐5. [PUBMED: 20473044] - PubMed
Davis 1999 {published data only}
-
- Davis JL, Smith RL. Painful peripheral diabetic neuropathy treated with venlafaxine HCl extended release capsules. Diabetes Care 1999;22(11):1909‐10. [PUBMED: 10546032] - PubMed
Durand 2002 {published data only}
-
- Durand JP, Goldwasser F. Dramatic recovery of paclitaxel‐disabling neurosensory toxicity following treatment with venlafaxine. Anti‐Cancer Drugs 2002;13(7):777‐80. [PUBMED: 12187335] - PubMed
Durand 2012 {published data only}
-
- Durand JP, Deplanque G, Montheil V, Gornet JM, Scotte F, Mir O, et al. Efficacy of venlafaxine for the prevention and relief of oxaliplatin‐induced acute neurotoxicity: results of EFFOX, a randomized, double‐blind, placebo‐controlled phase III trial. Annals of Oncology 2012;23(1):200‐5. - PubMed
Kadiroglu 2008 {published data only (unpublished sought but not used)}
-
- Kadiroglu AK, Sit D, Kayabasi H, Kemal TA, Tasdemir N, Yilmaz ME. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. Journal of Diabetes and its Complications 2008;22(4):241‐5. - PubMed
Kiayias 2000 {published data only}
-
- Kiayias JA, Vlachou ED, Lakka‐Papadodima E. Venlafaxine HCl in the treatment of painful peripheral diabetic neuropathy. Diabetes Care 2000;23(5):699. [PUBMED: 10834432] - PubMed
Lithner 2000 {published data only}
-
- Lithner F. Venlafaxine in treatment of painful peripheral diabetic neuropathy. Diabetes Care 2000;23(11):1710‐1. [PUBMED: 11092304] - PubMed
Pernia 2000 {published data only}
-
- Pernia A, Mico JA, Calderon E, Torres LM. Venlafaxine for the treatment of neuropathic pain. Journal of Pain and Symptom Management 2000;19(6):408‐10. [PUBMED: 10991644] - PubMed
Raskin 2006 {published data only}
-
- Raskin J, Smith TR, Wong K, Pritchett YL, D'Souza D, Iyengar S, et al. Duloxetine versus routine care in the long‐term management of diabetic peripheral neuropathic pain. Journal of Palliative Medicine 2006;9(1):29‐40. [PUBMED: 16430342] - PubMed
Rej 2014 {published data only}
Reuben 2004 {published data only}
-
- Reuben SS, Makari‐Judson G, Lurie SD. Evaluation of efficacy of the perioperative administration of venlafaxine XR in the prevention of post‐mastectomy pain syndrome. Journal of Pain and Symptom Management 2004;27(2):133‐9. [PUBMED: 15157037] - PubMed
Simpson 2001 {published data only}
-
- Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. Journal of Clinical Neuromuscular Disease 2001;3(2):53‐62. - PubMed
Sumpton 2001 {published data only}
-
- Sumpton JE, Moulin DE. Treatment of neuropathic pain with venlafaxine. Annals of Pharmacotherapy 2001;35(5):557‐9. [PUBMED: 11346061] - PubMed
Additional references
AUREF 2012
-
- PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 22 January 2013).
Bolden‐Watson 1993
-
- Bolden‐Watson C, Richelson E. Blockade by newly‐developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sciences 1993;52(12):1023‐9. [PUBMED: 8445992] - PubMed
Bouhassira 2008
Dharmshaktu 2012
-
- Dharmshaktu P, Tayal V, Singh KB. Efficacy of antidepressants as analgesics: a review. Journal of Clinical Pharmacology 2012;52:6‐17. [PUBMED: 21415285] - PubMed
Dworkin 2008
Enggaard 2001
Farrar 2001
-
- Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain 2011;94(2):149‐58. [PUBMED: 11690728] - PubMed
Gustorff 2008
Hall 2008
Hearn 2014
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Iannetti 2005
-
- Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, et al. Pharmacological modulation of pain‐related brain activity during normal and central sensitization states in humans. Proceedings of the National Academy of Sciences of the United States of America 2005;102:18195‐200. - PMC - PubMed
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: ] - PubMed
Jensen 2011
Katusic 1991
-
- Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota,1945‐1984. Neuroepidemiology 1991;10:276‐81. - PubMed
Koopman 2009
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
Lang 1996
Lee 2010
-
- Lee Y‐C, Chen P‐P. A review of SSRIs and SNRIs in neuropathic pain. Expert Opinion in Pharmacotherapy 2010;11(17):2813‐25. [PUBMED: 20642317] - PubMed
McQuay 1998
-
- McQuay H, Moore R. An Evidence‐Based Resource for Pain Relief. Oxford: Oxford University Press, 1998.
McQuay 2007
-
- McQuay HJ, Smith LA, Moore RA. Chronic pain. In: Stevens A, Raftery J, Mant J, Simpson S editor(s). Health Care Needs Assessment. 3rd Edition. Oxford: Radcliffe Publishing, 2007. [ISBN: 978‐1‐84619‐063‐6]
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978–0–931092–69–5]
Moore 2009a
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: ] - PMC - PubMed
Moore 2009b
Moore 2010a
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: ] - PubMed
Moore 2010b
Moore 2010c
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: ] - PMC - PubMed
Moore 2010d
-
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses ‐ do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151(3):592‐7. [DOI: ] - PubMed
Moore 2011a
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: ] - PubMed
Moore 2011b
Moore 2012
-
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265‐8. - PubMed
Moore 2014
Muth 1986
Muth 1991
-
- Muth EA, Moyer JA, Haskins JT. Biochemical, neurophysiological, and behavioral effects of Wy‐45,233 and other identified metabolites of the antidepressant venlafaxine. Drug Development Research 1991;23(2):191‐9. [DOI: 10.1002/ddr.430230210] - DOI
O'Brien 2010
Onghena 1992
Raftery 2011
-
- Raftery MN, Sarma K, Murphy AW, Harpe D, Normand C, McGuire BE. Chronic pain in the Republic of Ireland ‐ community prevalence, psychosocial profile and predictors of pain‐related disability: results from the Prevalence, Impact and Cost of Chronic Pain (PRIME) study, part 1. Pain 2011;152(5):1096‐103. [DOI: 10.1016/j.pain.2011.01.019; PUBMED: 21450402] - DOI - PubMed
Rappaport 1994
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Saarto 2007
Schreiber 1999
Seo 2010
-
- Seo HJ, Sohi MS, Patkar AA, Masand PS, Pae CU. Desvenlafaxine succinate: a newer antidepressant for the treatment of depression and somatic symptoms. Postgraduate Medicine 2010;122(1):125‐38. - PubMed
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010
Sultan 2008
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical