Benefits of Systemic Anti-inflammatory Therapy versus Fluocinolone Acetonide Intraocular Implant for Intermediate Uveitis, Posterior Uveitis, and Panuveitis: Fifty-four-Month Results of the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study
- PMID: 26298715
- PMCID: PMC4581989
- DOI: 10.1016/j.ophtha.2015.06.042
Benefits of Systemic Anti-inflammatory Therapy versus Fluocinolone Acetonide Intraocular Implant for Intermediate Uveitis, Posterior Uveitis, and Panuveitis: Fifty-four-Month Results of the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study
Abstract
Purpose: To compare the benefits of fluocinolone acetonide implant therapy versus systemic corticosteroid therapy supplemented (when indicated) with immunosuppression for intermediate uveitis, posterior uveitis, and panuveitis.
Design: Additional follow-up of a randomized comparative effectiveness trial cohort.
Participants: Two hundred fifty-five patients with intermediate uveitis, posterior uveitis, or panuveitis randomized to implant or systemic therapy.
Main outcome measures: Best-corrected visual acuity (BCVA), visual field mean deviation (MD), activity of uveitis, and presence of macular edema (per reading center grading) ascertained prospectively.
Methods: Trial participants were followed-up for 54 months from original randomization.
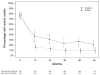
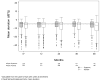
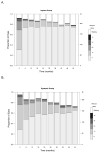
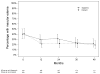
Results: The visual function trajectory in uveitic eyes demonstrated a similar (P = 0.73) degree of modest (not statistically significant) improvement from baseline to 54 months in both groups (mean improvement in BCVA at 54 months, 2.4 and 3.1 letters in the implant and systemic groups, respectively). Many had excellent initial visual acuity, limiting the potential for improvement. The mean automated perimetry MD score remained similar to baseline throughout 48 months of follow-up in both groups. Overall control of inflammation was superior in the implant group at every time point assessed (P < 0.016), although most eyes in the systemic therapy arm also showed substantial improvement, achieving complete control or low levels of inflammation. Although macular edema improved significantly more often with implant treatment within the first 6 months, the systemic group gradually improved over time such that the proportions with macular edema converged in the 2 groups by 36 months and overlapped thereafter (P = 0.41 at 48 months).
Conclusions: Visual outcomes of fluocinolone acetonide implant and systemic treatment for intermediate uveitis, posterior uveitis, and panuveitis were similarly favorable through 54 months. The implant maintained a clear advantage in controlling inflammation through 54 months. Nevertheless, with systemic therapy, most patients also experienced greatly improved inflammatory status. Macular edema improved equally with longer follow-up. Based on cost effectiveness and side-effect considerations, systemic therapy may be indicated as the initial treatment for many bilateral uveitis cases. However, implant therapy is a reasonable alternative, especially for unilateral cases and when systemic therapy is not feasible or is not successful.
Copyright © 2015 American Academy of Ophthalmology. All rights reserved.
Conflict of interest statement
Figures



References
-
- National Advisory Eye Council Program Planning Subcommittee. Vision Research—A National Plan: 1983–1987. Volume 1: The 1983 Report of the National Advisory Eye Council. Bethesda, MD: National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services; 1983. [Accessed November 13, 2014]. p. 13. Available at: http://ia700402.us.archive.org/34/items/visionresearchna01nati/visionres....
-
- Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–8. - PubMed
-
- Rathinam SR, Namperumalsamy P. Global variation and pattern changes in epidemiology of uveitis. Indian J Ophthalmol. 2007;55:173–83. - PubMed
-
- ten Doesschate J. Causes of blindness in The Netherlands. Doc Ophthalmol. 1982;52:279–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

