Neuronal migration disorders: Focus on the cytoskeleton and epilepsy
- PMID: 26299390
- PMCID: PMC6508100
- DOI: 10.1016/j.nbd.2015.08.003
Neuronal migration disorders: Focus on the cytoskeleton and epilepsy
Abstract
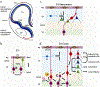
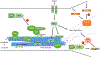
A wide spectrum of focal, regional, or diffuse structural brain abnormalities, collectively known as malformations of cortical development (MCDs), frequently manifest with intellectual disability (ID), epilepsy, and/or autistic spectrum disorder (ASD). As the acronym suggests, MCDs are perturbations of the normal architecture of the cerebral cortex and hippocampus. The pathogenesis of these disorders remains incompletely understood; however, one area that has provided important insights has been the study of neuronal migration. The amalgamation of human genetics and experimental studies in animal models has led to the recognition that common genetic causes of neurodevelopmental disorders, including many severe epilepsy syndromes, are due to mutations in genes regulating the migration of newly born post-mitotic neurons. Neuronal migration genes often, though not exclusively, code for proteins involved in the function of the cytoskeleton. Other cellular processes, such as cell division and axon/dendrite formation, which similarly depend on cytoskeletal functions, may also be affected. We focus here on how the susceptibility of the highly organized neocortex and hippocampus may be due to their laminar organization, which involves the tight regulation, both temporally and spatially, of gene expression, specialized progenitor cells, the migration of neurons over large distances and a birthdate-specific layering of neurons. Perturbations in neuronal migration result in abnormal lamination, neuronal differentiation defects, abnormal cellular morphology and circuit formation. Ultimately this results in disorganized excitatory and inhibitory activity leading to the symptoms observed in individuals with these disorders.
Keywords: Cortical malformation; Molecular and cellular mechanisms; Mouse model; Neurodevelopment.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Alkuraya FS, Cai X, Emery C, Mochida GH, Al-Dosari MS, Felie JM, Hill RS, Barry BJ, Partlow JN, Gascon GG, Kentab A, Jan M, Shaheen R, Feng Y, Walsh CA, 2011. Human mutations in NDE1 cause extreme microcephaly with lissencephaly [corrected]. American journal of human genetics 88, 536–547. - PMC - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL, 1997. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science (New York, N.Y.) 278, 474–476. - PubMed
-
- Anton ES, Marchionni MA, Lee KF, Rakic P, 1997. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124, 3501–3510. - PubMed
-
- Arimura N, Kaibuchi K, 2007. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nature reviews. Neuroscience 8, 194–205. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

