Surfactant therapy for bronchiolitis in critically ill infants
- PMID: 26299681
- PMCID: PMC7104667
- DOI: 10.1002/14651858.CD009194.pub3
Surfactant therapy for bronchiolitis in critically ill infants
Abstract
Background: Bronchiolitis is one of the most frequent causes of respiratory failure in infants; some infants will require intensive care and mechanical ventilation. There is lack of evidence regarding effective treatment for bronchiolitis other than supportive care. Abnormalities of surfactant quantity or quality (or both) have been observed in severe cases of bronchiolitis. Exogenous surfactant administration appears to favourably change the haemodynamics of the lungs and may be a potentially promising therapy for severe bronchiolitis. This is an update of a review published in Issue 9, 2012. We did not identify any new studies for inclusion, and our conclusions remain unchanged.
Objectives: To evaluate the efficacy of exogenous surfactant administration (i.e. intratracheal administration of surfactant of any type (whether animal-derived or synthetic), at any dose and at any time after start of ventilation) compared to placebo, no intervention or standard care in reducing mortality and the duration of ventilation in infants and children with bronchiolitis requiring mechanical ventilation.
Search methods: We searched the Cochrane Central Register of Controlled Studies (CENTRAL; 2015, Issue 5) which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (1948 to June week 3, 2015); EMBASE (1974 to June 2015); CINAHL (1982 to June 2015); LILACS (1985 to June 2015); and Web of Science (1985 to June 2015).
Selection criteria: We considered prospective, randomised controlled trials (RCTs) and quasi-RCTs evaluating the effect of exogenous surfactant in infants and children with bronchiolitis requiring mechanical ventilation.
Data collection and analysis: Two review authors selected studies independently. We extracted the data using a predefined proforma, independently analysed the data, and performed meta-analyses.
Main results: We included three small RCTs enrolling 79 participants. Two trials did not use a placebo in the control arms and the third trial used air placebo. Two included studies reported no mortality. We judged all three of the included studies to be at low risk or unclear risk across all risk of bias categories; we did not judge any of the studies to be at high risk of bias in any category. Our pooled analysis of the three trials revealed that duration of mechanical ventilation was not significantly different between the groups (mean difference (MD) -63.04, 95% confidence interval (CI) -130.43 to 4.35 hours) but duration of intensive care unit (ICU) stay was less in the surfactant group compared to the control group: MD -3.31, 95% CI -6.38 to -0.25 days. After excluding one trial which produced significant heterogeneity, the duration of mechanical ventilation and duration of ICU stay were significantly lower in the surfactant group compared to the control group: MD -28.99, 95% CI -40.10 to -17.87 hours; and MD -1.81, 95% CI -2.42 to -1.19 days, respectively. Use of surfactant had favourable effects on oxygenation and CO2 elimination. No adverse effects and no complications were observed in any of the three included studies. The level of evidence for duration of mechanical ventilation, duration of intensive care unit stay, oxygenation parameters, and carbon dioxide parameters was of moderate quality.
Authors' conclusions: Use of surfactant had favourable effects on duration of mechanical ventilation, duration of ICU stay, oxygenation, and CO2 elimination. However, the studies are few and small (n = 79) so available evidence is insufficient to establish the effectiveness of surfactant therapy for bronchiolitis in critically ill infants who require mechanical ventilation. There is a need for larger trials with adequate power and a cost-effectiveness analysis to evaluate the effectiveness of exogenous surfactant therapy for infants with bronchiolitis who require intensive care management.
Conflict of interest statement
Kana R Jat: none known. Deepak Chawla: none known.
Figures



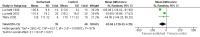
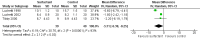









Update of
-
Surfactant therapy for bronchiolitis in critically ill infants.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD009194. doi: 10.1002/14651858.CD009194.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Aug 24;(8):CD009194. doi: 10.1002/14651858.CD009194.pub3. PMID: 22972138 Updated.
References
References to studies included in this review
Luchetti 1998 {published data only}
-
- Luchetti M, Casiraghi G, Valsecchi R, Galassini E, Marraro G. Porcine‐derived surfactant treatment of severe bronchiolitis. Acta Anaesthesiologica Scandinavica 1998;42(7):805‐10. - PubMed
Luchetti 2002 {published data only}
-
- Luchetti M, Ferrero F, Gallini C, Natale A, Pigna A, Tortorolo L, et al. Multicenter, randomized, controlled study of porcine surfactant in severe respiratory syncytial virus‐induced respiratory failure. Pediatric Critical Care Medicine 2002;3(3):261‐8. - PubMed
Tibby 2000 {published data only}
-
- Tibby SM, Hatherill M, Wright SM, Wilson P, Postle AD, Murdoch IA. Exogenous surfactant supplementation in infants with respiratory syncytial virus bronchiolitis. American Journal of Respiratory and Critical Care Medicine 2000;162(4 Pt 1):1251‐6. - PubMed
References to studies excluded from this review
Moller 2003 {published data only}
Willson 1999 {published data only}
-
- Willson DF, Zaritsky A, Bauman LA, Dockery K, James RL, Conrad D, et al. Members of the Mid‐Atlantic Pediatric Critical Care Network. Instillation of calf lung surfactant extract (calfactant) is beneficial in pediatric acute hypoxemic respiratory failure. Critical Care Medicine 1999;27(1):188‐95. - PubMed
Willson 2005 {published data only}
-
- Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA 2005;293(4):470‐6. - PubMed
Additional references
Ardell 2015
Barr 2000
-
- Barr FE, Pedigo H, Johnson TR, Shepherd VL. Surfactant protein‐A enhances uptake of respiratory syncytial virus by monocytes and U937 macrophages. American Journal of Respiratory Cell and Molecular Biology 2000;23(5):586‐92. - PubMed
CDC 2010
-
- Centers for Disease Control and Prevention. Respiratory Syncytial Virus. www.cdc.gov/rsv/ (accessed 15 February 2011).
Dargaville 1996
Davison 2004
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Farley 2014
Fernandes 2013
Gadomski 2014
Goerke 1998
-
- Goerke J. Pulmonary surfactant: functions and molecular composition. Acta Biochimica et Biophysica 1998;1408(2‐3):79‐89. - PubMed
GRADEproGDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Version 25th June 2015. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Griese 1999
-
- Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. European Respiratory Journal 1999;13(6):1455‐76. - PubMed
Handforth 2000
-
- Handforth J, Friedland JS, Sharland M. Basic epidemiology and immunopathology of RSV in children. Paediatric Respiratory Reviews 2000;1:210‐4. - PubMed
Hartling 2011a
Hartling 2011b
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holman 2003
-
- Holman RC, Shay DK, Curns AT, Lingappa JR, Anderson LJ. Risk factors for bronchiolitis‐associated deaths among infants in the United States. Pediatric Infectious Disease Journal 2003;22(6):483‐90. - PubMed
Kabir 2003
-
- Kabir ML, Haq N, Hoque M, Ahmed F, Amin R, Hossain A, et al. Evaluation of hospitalized infants and young children with bronchiolitis ‐ a multi centre study. Mymensingh Medical Journal 2003;12:128‐33. - PubMed
Kerr 1999
-
- Kerr MH, Paton JY. Surfactant protein levels in severe respiratory syncytial virus infection. American Journal of Respiratory and Critical Care Medicine 1999;159(4 Pt 1):1115‐8. - PubMed
Lahti 2002
-
- Lahti M, Lofgren J, Marttila R, Renko M, Klaavuniemi T, Haataja R, et al. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatric Research 2002;51(6):696‐9. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Levy 1997
-
- Levy BT, Graber MA. Respiratory syncytial virus infection in infants and young children. Journal of Family Practice 1997;45(6):473‐81. - PubMed
Liet 2010
Logan 2009
Löfgren 2002
-
- Löfgren J, Rämet M, Renko M, Marttila R, Hallman M. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. Journal of Infectious Diseases 2002;185(3):283‐9. - PubMed
Meates‐Dennis 2005
-
- Meates‐Dennis M. Bronchiolitis. Archives of Disease in Childhood Education and Practice Edition 2005;90:81–6.
Meissner 2009
-
- Meissner HC, Bocchini JA. Reducing RSV hospitalizations AAP modifies recommendations for use of palivizumab in high‐risk infants, young children. AAP News 2009;30(7):1.
Mercus 2001
-
- Merkus PJ, de Hoog M, van Gent R, de Jongste JC. DNase treatment for atelectasis in infants with severe respiratory syncytial virus bronchiolitis. European Respiratory Journal 2001;18(4):734‐7. - PubMed
Moher 2009
Nasr 2001
-
- Nasr SZ, Strouse PJ, Soskolne E, Maxvold NJ, Garver KA, Rubin BK, et al. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest 2001;120(1):203‐8. - PubMed
Navas 1992
-
- Navas L, Wang E, Carvalho V, Robinson J. Improved outcome of respiratory syncytial virus infection in a high‐risk hospitalized population of Canadian children. Pediatric Investigators Collaborative Network on Infections in Canada. Journal of Pediatrics 1992;121(3):348‐54. - PubMed
Panickar 2005
Possmayer 1990
-
- Possmayer F. The role of surfactant‐associated proteins. American Review of Respiratory Disease 1990;142(4):749‐52. - PubMed
Ramanathan 2009
-
- Ramanathan R. Animal‐derived surfactants: where are we? The evidence from randomized, controlled clinical trials. Journal of Perinatology 2009;29(Suppl 2):38‐43. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roqué i Figuls 2012
Seger 2009
Shay 2001
-
- Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979‐1997. Journal of Infectious Diseases 2001;183(1):16‐22. - PubMed
Skelton 1999
-
- Skelton R, Holland P, Darowski M, Chetcuti PA, Morgan LW, Harwood JL. Abnormal surfactant composition and activity in severe bronchiolitis. Acta Paediatrica 1999;88(9):942‐6. - PubMed
Soll 1998
Umoren 2011
Ventre 2007
Wood 1993
-
- Wood AJJ, Jobe AH. Pulmonary surfactant therapy. New England Journal of Medicine 1993;328(12):861‐8. - PubMed
Wright 2003
References to other published versions of this review
Jat 2011
-
- Jat KR, Chawla D. Surfactant therapy for bronchiolitis in critically ill infants. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009194] - DOI
Jat 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

