The TWEAK receptor Fn14 is a potential cell surface portal for targeted delivery of glioblastoma therapeutics
- PMID: 26300004
- PMCID: PMC4850525
- DOI: 10.1038/onc.2015.310
The TWEAK receptor Fn14 is a potential cell surface portal for targeted delivery of glioblastoma therapeutics
Abstract
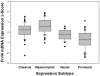
Fibroblast growth factor-inducible 14 (Fn14; TNFRSF12A) is the cell surface receptor for the tumor necrosis factor (TNF) family member TNF-like weak inducer of apoptosis (TWEAK). The Fn14 gene is normally expressed at low levels in healthy tissues but expression is significantly increased after tissue injury and in many solid tumor types, including glioblastoma (GB; formerly referred to as 'GB multiforme'). GB is the most common and aggressive primary malignant brain tumor and the current standard-of-care therapeutic regimen has a relatively small impact on patient survival, primarily because glioma cells have an inherent propensity to invade into normal brain parenchyma, which invariably leads to tumor recurrence and patient death. Despite major, concerted efforts to find new treatments, a new GB therapeutic that improves survival has not been introduced since 2005. In this review article, we summarize studies indicating that (i) Fn14 gene expression is low in normal brain tissue but is upregulated in advanced brain cancers and, in particular, in GB tumors exhibiting the mesenchymal molecular subtype; (ii) Fn14 expression can be detected in glioma cells residing in both the tumor core and invasive rim regions, with the maximal levels found in the invading glioma cells located within normal brain tissue; and (iii)
Tweak: Fn14 engagement as well as Fn14 overexpression can stimulate glioma cell migration, invasion and resistance to chemotherapeutic agents in vitro. We also discuss two new therapeutic platforms that are currently in development that leverage Fn14 overexpression in GB tumors as a way to deliver cytotoxic agents to the glioma cells remaining after surgical resection while sparing normal healthy brain cells.
Conflict of interest statement
Figures




References
-
- Chicheportiche Y, Bourdon PR, Xu H, Hsu Y, Scott H, Hession C, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–10. - PubMed
-
- Meighan-Mantha RL, Hsu DKW, Guo Y, Brown SAN, Feng SY, Peifley KA, et al. The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem. 1999;274:33166–76. - PubMed
-
- Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, Lindner V, et al. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15:837–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

