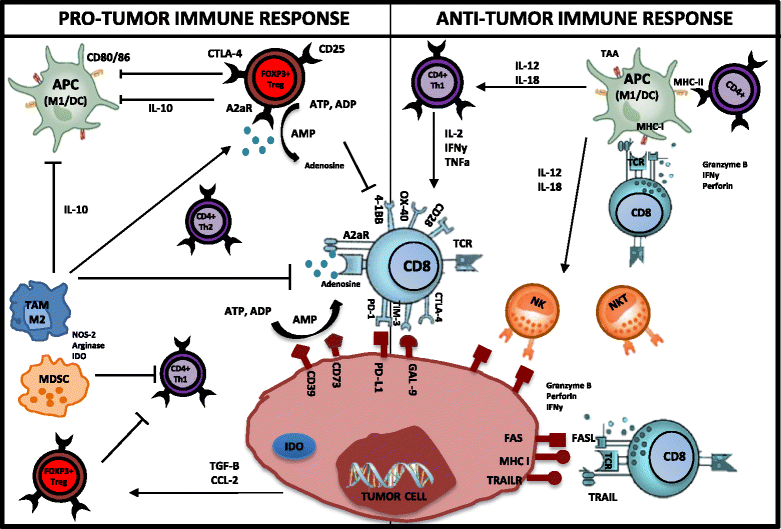
Fig. 1
Interactions between the immune microenvironment and tumor cells in breast cancer. The antitumor immune response is dependent upon CD4+ (Th1) IFNγ production, which in turn mediates the expansion, differentiation, and activation of tumor-specific CD8+. CD8+ cytotoxic T cells induce cell lysis via recognition of specific TAAs such as MHC, FAS, and TRAILR on the surface of cancer cells/APCs. Similarly, CD4+ T cells are able to recognize MHC II on APCs. As a result of this complex formation (TCR-MHC/Peptide), high levels of granzymes, IFNγ, and perforin are released from CTLs, resulting in granule exocytosis and tumor cell death via apoptosis. NK and NKT cells with the help of APCs (DCs/M1) and CD4+Th1 are able to recognize and eliminate tumor cells. In the pro-tumor environment, CTLA-4, TIM-3, and PD-1 deliver inhibitory signals as a result of T-cell exhaustion/anergy caused by prolonged activation. CTLA-4 negatively regulates T-cell activation during the ‘priming’ phase of T-cell response. PD-1 expressed on T cells in the effector phase of T-cell response binds to its ligand PD-L1, expressed within the tumor microenvironment. This results in inhibition of T-cell activity (apoptosis). FOXP3+ Treg cells play a critical role during the selection of high-avidity CD8+ T cells, reducing their functionality. Tregs also have inhibitory action on APCs, CD8+ T cells, NKs, and CD4+ Th1 T cells. Both Tregs and tumor cells produce adenosine, which has inhibitory effects on T cells. Tumor cells can secrete cytokines and chemokines (e.g., TGF-β, CCL2) that recruit and stimulate suppressive cells such as Tregs, MDSCs, and M2 macrophages. M2 macrophages and MDSCs inhibit T-cell responses through nutrient sequestration via arginase, ROS, and NOS generation, as well as interference with trafficking into the tumor site. The upregulation of immunosuppressive enzymes such as IDO and arginase catabolizes essential nutrients required for effector cell activation. Furthermore, tumor cells downregulate MHC molecules, lose expression of antigenic molecules, and upregulate inhibitory molecules such as PD-L1, causing immune recognition to be inhibited and thus allowing immune escape and cancer progression. This figure was made exclusively for this manuscript. A2aR A2A adenosine receptor, ADP adenosine diphosphate, AMP adenosine monophosphate, APC antigen-presenting cell, ATP adenosine triphosphate, CCl-2 chemokine ligand-2, CTL cytotoxic T lymphocyte, CTLA-4 cytotoxic t lymphocyte-associated protein, DC dendritic cell, FAS fatty-acid synthase, GAL-9 galectin-9, IDO indolamine 2,3-dioxygenase, IFNγ interferon gamma, IL interleukin, M1/M1 TAM tumor-associated macrophage, MDSC myeloid-derived suppressor cell, MHC major histocompatibility complex, NK natural killer, NKT natural killer T cell, NOS nitric oxide synthase, PD-1 programmed death, ROS reactive oxygen species, TAA tumor-associated antigen, TCR T-cell receptor, TGF-β transforming growth factor beta, TNFα tumor necrosis factor alpha, TRAIL TNF-related apoptosis-inducing ligand, Treg T regulatory cell