Ancient Origin of cGAS-STING Reveals Mechanism of Universal 2',3' cGAMP Signaling
- PMID: 26300263
- PMCID: PMC4575873
- DOI: 10.1016/j.molcel.2015.07.022
Ancient Origin of cGAS-STING Reveals Mechanism of Universal 2',3' cGAMP Signaling
Abstract
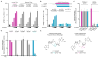
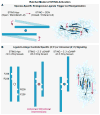
In humans, the cGAS-STING immunity pathway signals in response to cytosolic DNA via 2',3' cGAMP, a cyclic dinucleotide (CDN) second messenger containing mixed 2'-5' and 3'-5' phosphodiester bonds. Prokaryotes also produce CDNs, but these are exclusively 3' linked, and thus the evolutionary origins of human 2',3' cGAMP signaling are unknown. Here we illuminate the ancient origins of human cGAMP signaling by discovery of a functional cGAS-STING pathway in Nematostella vectensis, an anemone species >500 million years diverged from humans. Anemone cGAS appears to produce a 3',3' CDN that anemone STING recognizes through nucleobase-specific contacts not observed in human STING. Nevertheless, anemone STING binds mixed-linkage 2',3' cGAMP indistinguishably from human STING, trapping a unique structural conformation not induced by 3',3' CDNs. These results reveal that human mixed-linkage cGAMP achieves universal signaling by exploiting a deeply conserved STING conformational intermediate, providing critical insight for therapeutic targeting of the STING pathway.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




References
-
- Chen H, Sun H, You F, Sun W, Zhou X, Chen L, Yang J, Wang Y, Tang H, Guan Y, et al. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147:436–446. - PubMed
-
- Chin KH, Tu ZL, Su YC, Yu YJ, Chen HC, Lo YC, Chen CP, Barber GN, Chuah ML, Liang ZX, et al. Novel c-di-GMP recognition modes of the mouse innate immune adaptor protein STING. Acta crystallographica Section D, Biological crystallography. 2013;69:352–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

