GABAA receptor activity shapes the formation of inhibitory synapses between developing medium spiny neurons
- PMID: 26300728
- PMCID: PMC4526800
- DOI: 10.3389/fncel.2015.00290
GABAA receptor activity shapes the formation of inhibitory synapses between developing medium spiny neurons
Abstract
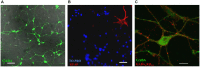
Basal ganglia play an essential role in motor coordination and cognitive functions. The GABAergic medium spiny neurons (MSNs) account for ~95% of all the neurons in this brain region. Central to the normal functioning of MSNs is integration of synaptic activity arriving from the glutamatergic corticostriatal and thalamostriatal afferents, with synaptic inhibition mediated by local interneurons and MSN axon collaterals. In this study we have investigated how the specific types of GABAergic synapses between the MSNs develop over time, and how the activity of GABAA receptors (GABAARs) influences this development. Isolated embryonic (E17) MSNs form a homogenous population in vitro and display spontaneous synaptic activity and functional properties similar to their in vivo counterparts. In dual whole-cell recordings of synaptically connected pairs of MSNs, action potential (AP)-activated synaptic events were detected between 7 and 14 days in vitro (DIV), which coincided with the shift in GABAAR operation from depolarization to hyperpolarization, as detected indirectly by intracellular calcium imaging. In parallel, the predominant subtypes of inhibitory synapses, which innervate dendrites of MSNs and contain GABAAR α1 or α2 subunits, underwent distinct changes in the size of postsynaptic clusters, with α1 becoming smaller and α2 larger over time, while both the percentage and the size of mixed α1/α2-postsynaptic clusters were increased. When activity of GABAARs was under chronic blockade between 4-7 DIV, the structural properties of these synapses remained unchanged. In contrast, chronic inhibition of GABAARs between 7-14 DIV led to reduction in size of α1- and α1/α2-postsynaptic clusters and a concomitant increase in number and size of α2-postsynaptic clusters. Thus, the main subtypes of GABAergic synapses formed by MSNs are regulated by GABAAR activity, but in opposite directions, and thus appear to be driven by different molecular mechanisms.
Keywords: GABAA receptor depolarization; GABAA receptors; GABAergic synapses; hyperpolarizing shift; striatal medium spiny neuron.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

