Maternal immune activation evoked by polyinosinic:polycytidylic acid does not evoke microglial cell activation in the embryo
- PMID: 26300736
- PMCID: PMC4525016
- DOI: 10.3389/fncel.2015.00301
Maternal immune activation evoked by polyinosinic:polycytidylic acid does not evoke microglial cell activation in the embryo
Abstract
Several studies have indicated that inflammation during pregnancy increases the risk for the development of neuropsychiatric disorders in the offspring. Morphological brain abnormalities combined with deviations in the inflammatory status of the brain can be observed in patients of both autism and schizophrenia. It was shown that acute infection can induce changes in maternal cytokine levels which in turn are suggested to affect fetal brain development and increase the risk on the development of neuropsychiatric disorders in the offspring. Animal models of maternal immune activation reproduce the etiology of neurodevelopmental disorders such as schizophrenia and autism. In this study the poly (I:C) model was used to mimic viral immune activation in pregnant mice in order to assess the activation status of fetal microglia in these developmental disorders. Because microglia are the resident immune cells of the brain they were expected to be activated due to the inflammatory stimulus. Microglial cell density and activation level in the fetal cortex and hippocampus were determined. Despite the presence of a systemic inflammation in the pregnant mice, there was no significant difference in fetal microglial cell density or immunohistochemically determined activation level between the control and inflammation group. These data indicate that activation of the fetal microglial cells is not likely to be responsible for the inflammation induced deficits in the offspring in this model.
Keywords: cortex; embryo; maternal immune activation; microglia; neuropsychiatric disorders.
Figures

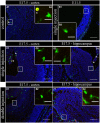
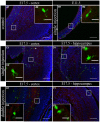
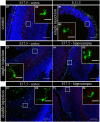
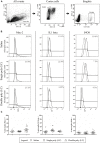
Similar articles
-
Role of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation and Subsequent Immune Challenge in the Behaviour and Microglial Cell Trajectory in Adult Offspring: A Study of the Neurodevelopmental Model of Schizophrenia.Int J Mol Sci. 2021 Feb 4;22(4):1558. doi: 10.3390/ijms22041558. Int J Mol Sci. 2021. PMID: 33557113 Free PMC article.
-
Maternal immune activation-induced PPARγ-dependent dysfunction of microglia associated with neurogenic impairment and aberrant postnatal behaviors in offspring.Neurobiol Dis. 2019 May;125:1-13. doi: 10.1016/j.nbd.2019.01.005. Epub 2019 Jan 17. Neurobiol Dis. 2019. PMID: 30659984
-
The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model.Brain Behav Immun. 2014 Nov;42:138-46. doi: 10.1016/j.bbi.2014.06.013. Epub 2014 Jun 26. Brain Behav Immun. 2014. PMID: 24973728
-
Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders.Brain Behav Immun. 2018 Oct;73:51-65. doi: 10.1016/j.bbi.2018.06.001. Epub 2018 Jun 2. Brain Behav Immun. 2018. PMID: 29870753 Review.
-
Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders.Exp Neurol. 2018 Jan;299(Pt A):241-251. doi: 10.1016/j.expneurol.2017.07.002. Epub 2017 Jul 8. Exp Neurol. 2018. PMID: 28698032 Free PMC article. Review.
Cited by
-
N-acetylcysteine attenuates lipopolysaccharide-induced impairment in lamination of Ctip2-and Tbr1- expressing cortical neurons in the developing rat fetal brain.Sci Rep. 2016 Aug 31;6:32373. doi: 10.1038/srep32373. Sci Rep. 2016. PMID: 27577752 Free PMC article.
-
Maternal Type-I interferon signaling adversely affects the microglia and the behavior of the offspring accompanied by increased sensitivity to stress.Mol Psychiatry. 2020 May;25(5):1050-1067. doi: 10.1038/s41380-019-0604-0. Epub 2019 Nov 26. Mol Psychiatry. 2020. PMID: 31772304 Free PMC article.
-
Microglia sequelae: brain signature of innate immunity in schizophrenia.Transl Psychiatry. 2022 Nov 28;12(1):493. doi: 10.1038/s41398-022-02197-1. Transl Psychiatry. 2022. PMID: 36443303 Free PMC article. Review.
-
New insight in the cross-talk between microglia and schizophrenia: From the perspective of neurodevelopment.Front Psychiatry. 2023 Feb 16;14:1126632. doi: 10.3389/fpsyt.2023.1126632. eCollection 2023. Front Psychiatry. 2023. PMID: 36873215 Free PMC article. Review.
-
Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function.Front Immunol. 2018 Apr 13;9:698. doi: 10.3389/fimmu.2018.00698. eCollection 2018. Front Immunol. 2018. PMID: 29706957 Free PMC article. Review.
References
-
- Anderson S. A., Marin O., Horn C., Jennings K., Rubenstein J. L. (2001). Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 128 353–363. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

